
A comparative study of high-pressure processing and microwave pasteurisation on the formation of hydroxymethylfurfural in stingless bee (Heterotrigona itama) honey
Introduction
Stingless bee honey is an effective wound healer used years ago (1). As a wound healer, stingless bee honey naturally possesses several significant properties that are very useful as wound dressing. Pimentel et al. [2022] (2) recently reviewed the nutraceutical and medicinal health-promoting effects of stingless bee honey by means of both in vitro and in vivo studies. The vital properties in the wound healing process are antimicrobial, antioxidant, and anti-inflammatory properties (1). Antimicrobial properties are crucial to prevent or avoid the infection of the injured area. As stingless bee honey is acidic, which is due to the organic acid content (0.57%) in the honey, it can deal with most microorganisms that survive at pH between 7.2 and 7.4 (3). According to Chanchao [2009] (4), there are three unique properties for stingless bee honey to act as an antimicrobial agent, which are the low pH value as the inhibitor to all acidophiles, a strong hyperosmotic effect due to high sugar saturation, and the presence of either hydrogen peroxidase as a by-product of glucose oxidation from glucose oxidase to gluconic acid or the presence of antimicrobial peptides. Molan [1999] (5) is in agreement with Chanchao [2009] (4) that another possible factor is the phytochemical factor, which is due to the presence of complex phenols and organic acids or known also as flavonoids. Flavonoids are unaffected by heat or light treatment and provide antibacterial agents even after honey is treated.
There are some toxic compounds that can be detected in honey. It is important to determine these compounds for the safety and quality of honey to be consumed. Trace elements and chemical compositions of honey and propolis have been investigated by researchers in their published works (6-9). Hydroxymethylfurfural (HMF) is one of the pertinent compounds commonly known in the honey industry as HMF concentration is recognised as a honey freshness indicator, and this compound is typically absent in fresh honey or present in relatively small amounts (10). HMF is a cyclic aldehyde, and it is a by-product of sugar degradation, mainly simple sugars (e.g., glucose and fructose) through a non-enzymatic browning reaction (Maillard reaction) during prolonged storage of honey or food processing (10). The authors also added that Malaysian Standard MS 2683 [2017] (11) has set the limit of HMF value in stingless bee honey not to exceed 30 mg/kg for both unprocessed and processed honey. However, the value tends to increase during processing and long storage time.
Mouhoubi-Tafinine et al. [2018] (12) reported that the HMF content of Algerian honey increased and exceeded the permissible value of 40 mg/kg (13) after storage at 35 ℃ for 9 months. Khalil et al. [2010] (14) showed that the HMF levels for all samples of Malaysian honey (Tualang, Gelam, Borneo tropical honey, and Manuka) increased after one year of storage at room temperature, which were evaluated by the comparison between three recommended methods for HMF analysis by the International Honey Commission (IHC) (15). A study on the physicochemical characteristics of Malaysian stingless bee honey from Trigona spp. exhibited HMF content ranged from 0.08±0.16 to 3.42±1.03 mg/kg, which is relatively in small amount (16). Both studies by Biluca et al. [2016] (17) and De Sousa et al. [2016] (18) reported the absence of HMF in their samples. Another factor affecting HMF content is the climate, where fresh honey from tropical regions usually contains HMF as the honey is exposed to the high surrounding temperature and the long period before harvesting (19,20).
At the same time, any treatment involving heating and high temperature of honey tends to influence HMF formation. Cozmuta et al. [2011] (21) studied the effect of thermal processing on the quality of polyfloral honey, and the results showed that at 50 ℃, the first 0.5 h of heating gave the lowest HMF formation ratio (i.e., 0%), and the value increased to 47.19% after 3 h of heating. The authors claimed that HMF formation is equally influenced by temperature and heating time. However, the findings of Turhan et al. [2008] (22) contradicted the previous statement, as heating floral honey and honeydew honey at 90 ℃ for up to 90 and 75 min, respectively, did not significantly increase HMF, and the value remained below the threshold value of 40 mg/kg after treatment. The authors also concluded that primitive storage conditions contributed to more significant results of the excessive formation of HMF rather than overheating. Hebbar et al. [2003] (23) reported that heating of honey for a shorter time (15 s) at higher power intensity (16 W/g) was found to result in a low HMF value (3.8 mg/kg) and higher diastase activity (12.0). Therefore, HMF formation in honey can be controlled with the right temperature and heating time during heat treatment. In health aspects, HMF is claimed to have the ability to cause negative effects on human health, such as DNA-damaging, genotoxic, organotoxic, mutagenic, carcinogenic, and enzyme inhibitory effects (10). The authors also emphasised that despite the negative effects of HMF on human health, HMF also offers health benefits and acts as an antioxidant, anti-allergen, antisickling agent, and anticarcinogen. However, the discussions are still inconclusive with very limited studies at preclinical levels have been conducted. In the same article, it was reported that 30–150 mg HMF could be ingested by the human body on daily basis from any food product that has been consumed. However, no safe level of consumption has been clarified as it depends on the organ function of an individual to excrete HMF from their body. Therefore, the Codex Alimentarius Commission [2001] (13) has set the maximum limit for the HMF level in honey at 40 mg/kg to ensure that honey is safe for consumption and does not undergo extensive heating during honey processing.
Beekeepers across the world have used various processing methods to preserve honey while also improving its quality. Due to the unique qualities of honey, different processing methods may be required for different types of honey. For instance, honeybee honey’s processing involves preheating, straining, filtration, heating, cooling, and bottling (Subramanian et al. 2007) (24). As for honeybee honey which is known to be low in moisture content and high in viscosity, preheating would be necessary in order to liquify the honey and ease its handling and processing steps. By utilising new technology accessible in today’s environment, the methods employed can be either conventional or non-conventional. High-pressure processing (HPP) is one of the non-thermal food preservation methods that can deactivate vegetative spoilage microorganisms and destructive pathogens using high pressure and also induce a pasteurisation effect that can be applied to both solid and liquid foods with high moisture content (25). Muntean et al. [2016] (25) added that HPP can be applied at very high pressure (400–600 MPa) and mild temperature below 45 ℃ without breaking covalent bonds with a minimal effect on the food chemistry (texture, taste, nutritional value, and appearance) even though this treatment is lethal to microorganisms. HPP offers many advantages while at the same time is limited to certain aspects. The advantages of HPP are the inactivation of vegetative bacteria and spores at higher temperatures; the ability to preserve nutrients, flavours, and colours; non-toxic to food; shorter processing times; uniformly treated food; avoid the use of chemical preservatives; and giving positive consumer appeal (26).
Nowadays, HPP is widely used in the preservation of fruits and vegetable juices, jam or purees (27-29), meats (30,31), seafood (32,33), shellfish (34), and fish products (35,36). In honey processing, HPP is preferable as it does not involve heating that can deteriorate honey. In a study by Chaikham & Prangthip [2015] (37), high-pressure and ultrasonic processing significantly affected the antioxidative properties of honeybee samples by increasing the quantities of total phenols, flavonoids, and antioxidant capacity with an increase in pressure levels and processing times. They found that high-pressure treatment at 500 MPa for 20 min were the best conditions to enhance their longan flower honey antioxidant properties. Furthermore, HPP is also acquainted with and closely related to the processing alternative of Manuka honey. Akhmazillah et al. [2013] (38) studied the effect of HPP and the conventional thermal process on the total phenolic content (TPC) of Manuka honey. The TPC of the Manuka honey was found to increase by 47% compared to the untreated honey, and the most effective conditions were determined to be at 600 MPa for 10 min. HPP can facilitate the extractability of some amino acids, proteins, and antioxidants with a phenolic hydroxyl group, which results in the increase of TPC (39,40) as phenolic compounds consist of hydrogen bonds that can be exaggerated at very high pressure (41). A year later, Fauzi et al. [2014] (42) investigated the effect of HPP on Manuka honey with broad aspects, including antioxidant activity, preservation of colour, and flow behaviour of the honey. The antioxidant activity of the HPP-treated samples increased by 30% with no changes in the colour of the samples. Meanwhile, the flow behaviour of the honey was retained based on the shear-thinning behaviour before and after treatment. As for stingless bee honey, a recent study by Razali et al. [2019] (43) studied the effect of HPP on antioxidant, diastase activity, and colour of the honey. As proven in previous studies, the authors also discovered that the antioxidant properties of stingless bee honey increased by 3% at the treatment conditions of 600 MPa for 10 min. To date, there are no other works that have reported the effect of HPP on HMF formation and the physicochemical properties of stingless bee honey. Therefore, this study aimed to evaluate the effect of HPP on the formation of HMF and other physicochemical properties of stingless bee (Heterotrigona itama) honey. This study will contribute new knowledge to the processing methods and safety measures for producing a higher quality of stingless bee honey. We present the following article in accordance with the MDAR reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-13/rc).
Methods
Materials
Stingless bee honey from H. itama was freshly harvested from a stingless bee farm located at the University Agricultural Park, Universiti Putra Malaysia. First, 3 kg per batch of honey was collected in the glass jars, capped, sealed, and immediately stored in an icebox and transferred to the laboratory. The honey was stored at the cold temperature of 4±1 ℃ for one week prior to the experiments. Three batches of honey were harvested individually from 15–20 colonies of stingless bee honey (H. itama), and all analyses were duplicated for each batch. All the chemicals used in this study were of either analytical or general grade.
HPP and microwave pasteurisation of stingless bee honey
An HPP unit (Avure Technologies, Ohio, USA) was employed to treat stingless bee honey following the method by Akhmazillah et al. [2013] (38), where the honey was suggested to be treated at 600 MPa with a treatment time less than 40 min. About 100 g of honey was packed and sealed in polyethene (PET) pouches, which were sealed using an impulse sealer (PFS-300, SAMMI, China). Distilled water was used as the medium in the chamber. The honey samples were subjected to a static pressure of 600 MPa for 5, 10, 15, 20, and 30 minutes treatment time, excluding both pressure increasing time and decompression time. The process was conducted at ambient temperature, and a thermocouple was used to monitor the temperature within the pressure medium in the vessel where the samples were placed. The HPP unit was connected to a computer with software that could monitor the process through a control system. After treatment, packed honey samples were immediately analysed to observe the HPP treatment effect, and other samples were transferred into autoclaved glass jars and stored at room temperature (25±1 ℃, 60% RH).
Microwave pasteurisation (MW) was conducted using a microwave (Panasonic NN J993, Panasonic, Japan) following the method by De La Paz Moliné et al. [2015] (44) and Ghazali et al. [1994] (45) for microwave heating of starfruit honey with modifications, where the samples were considered pasteurised if the temperature of the sample achieved 71 ℃. In the preliminary study, 100 mL of stingless bee honey was placed in a 150 mL beaker and microwaved at 700 W, and the time was measured gradually until the sample reached 71 ℃. The temperature of the honey was immediately measured and recorded right after being heated in the microwave by using a thermometer. Therefore, it was found that the time taken for the honey sample to reach the pasteurisation effect was 80 seconds. Then, all samples were treated with that optimised conditions throughout the study. All samples were placed in an incubator (Wisecube WIS-20 Daihan Scientific, Malaysia) at a temperature of 25 ℃ and analysed every week for up to a month. As this is an initial study, the storage stability study was conducted for only one month. Monitoring property changes in a longer period to consolidate the variation was recommended for future study.
Physicochemical analyses
All physicochemical analyses were performed following the established method described by Baroyi et al. [2019] (46) and Mohamad Ghazali et al. [2021] (47) for stingless bee honey. Moisture content (48) was analysed using the refractometry method by employing a refractometer (ABBE Refractometer AR2008, A. Kruss Optronic, Hamburg, Germany) at 25 ℃. Total soluble sugar value was used to determine the equivalent moisture content of honey using Eq. [1] (48):
Where RI is the refractive index.
Meanwhile, pH and free acidity were measured using the method described by Bogdanov et al. [2002] (15). For the determination of pH, 10 g of honey was dissolved into 75 mL of deionised water. A pH meter (Milwaukee, Mi 805, USA) was used to measure the pH value of honey. On the other hand, free acidity was determined by titrating 40 mL of the solution with 0.1 M NaOH until the pH achieved a value of 8.3 (the end point of phenolphthalein). The volume of NaOH used in the titration was recorded. The free acidity was expressed as equivalent acid per kg of honey (meq/kg) (15).
A rheometer (AR-G2, TA Instrument, New Castle, DE, USA) was employed to the measure viscosity (49) of honey for different processing methods and storage time at room temperature of 25 ℃, where the condition was maintained by means of a thermostat-controlled circulated water system. The equipment was set up with a 60 mm diameter plate geometry and 1° steel cone angle connected to a software (TA Instrument Advantage). Two grams of honey were used in a series of measurements that was conducted at a steady-state flow of shear rate of 1–1,000 s-1 and a plate gap of 1,000 µm. The viscosity of honey was obtained by the average value of 30 points.
Colour intensity was determined using a UV-VIS spectrophotometer (Ultrospec 3100 pro, Amersham Biosciences, Piscataway, NJ, USA). Colour intensity was determined by comparing the optical colour of double diluted honey with ultrapure water (PURELAB Flex 3, ELGA LabWAter, High Wycombe, UK) as the reference at 636 nm and categorised according to the classification based on the Pfund scale (mm Pfund =−38.7+371.4× Abs, where Abs = absorbance) described by Silvano et al. (2014) (50).
Sugar analysis
A 20% (w/v) honey solution in deionised water was prepared. Methanol was added to extract sugars. Mixtures were filtered through a 0.45 µm nylon syringe filter and injected into an HPLC system (Waters Alliance 2695, Waters Corporation, Milford, MA, USA). Sugar content (fructose, glucose, and maltose) was measured according to the IHC (15) and Malaysia Standard MS 2683 [2017] (11) methods. The HPLC employed was equipped with a refractive index detector (RID) (Waters 2414, Waters Corporation, Milford, MA, USA). The column used was LiChroCART-NH2 (250 mm × 4.6 mm, particle size 5 µm) (Merck, Darmstadt, Germany). The column temperature was fixed at 40 ℃. Twenty microliters of the filtered sample were injected into the system, and 80:20 (v/v) HPLC-grade acetonitrile and deionised water were used to elute the sugars in the samples isocratically at 1.5 mL/min. A standard curve of peak area versus concentration of sugar (0–5.0%, w/v) was plotted for each sugar standard (fructose, glucose, and maltose). The HPLC was validated with a precision of ±0.0005%.
HMF analysis
HMF content was determined using a method published by the IHC (15). Honey was diluted to 50% (w/v) with deionised water and filtered using a 0.45 µm nylon membrane filter and injected (20 µL) into an HPLC system (Waters Alliance 2695, Waters Corporation, Milford, MA, USA) equipped with a UV detector (Waters 2487, Waters Corporation, Milford, MA, USA). The column used was LiChroCART-RP 18 (250 mm × 4.6 mm, particle size 5 µm) (Merck, Darmstadt, Germany) while the detector was set to perform at 285 nm. The HMF content was separated and eluted using 90% (v/v) water-methanol as the mobile phase and flowed isocratically at 1.0 mL/min for 10 min of chromatographic run time. An HMF standard curve was plotted (0–100 µg/mL), and HMF content was calculated by comparing the corresponding peak areas of the samples with the standards. The HPLC was validated with a precision of ±0.0005%.
Statistical analysis
A one-way analysis of variance (ANOVA) of the data was performed using Tukey’s multiple comparison test to compare the means at a 95% confidence interval. A P≤0.05 was considered significant. All the analyses were performed using Minitab Statistical Software (Version 17, Minitab Inc., USA). All values reported were mean ± standard deviation.
Results
Effect of HPP and microwave pasteurisation on the physicochemical properties of stingless bee honey and storage stability
Moisture content
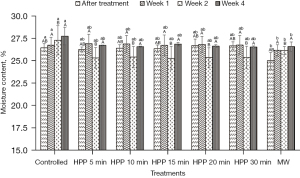
Figure 1 shows that the initial moisture content of the controlled honey at Week 0 is 26.45%±0.46%. At Week 0, the moisture content of honey was recorded right after HPP and MW. At the same time, the controlled honey was fresh and remained untreated. As the storage time increased, the moisture content of the controlled honey significantly (P≤0.05) increased. At Week 0, the moisture content of microwave-treated honey in this study significantly (P≤0.05) decreased to about 5.5% from the initial moisture content of fresh honey, and showed an increment after Week 1. However, the moisture content in honey was not significantly (P˃0.05) affected by HPP treatment. At the end of the storage study (Week 4), the moisture content of the controlled honey is the highest (27.75%±0.98%), while microwave-treated honey exhibited the lowest moisture content (26.58%±0.52%).
pH and free acidity
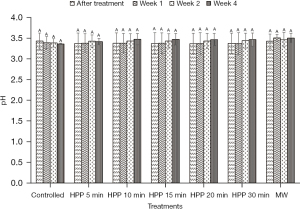
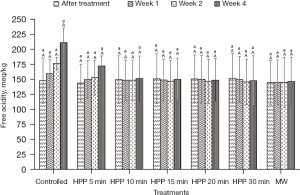
The results presented in Figure 2 and Figure 3 show that the values of pH and free acidity of the controlled stingless bee honey are 3.43±0.19 and 148±48 meq/kg, respectively. In this present work, the pH value of the honey is not significantly (P>0.05) affected regardless of the type of treatment and storage time subjected to the honey. However, the free acidity of the controlled honey increased from Week 1 until Week 4 with a percentage of 43%.
Viscosity
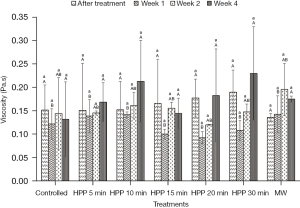
Figure 4 shows that the initial viscosity of the fresh honey used in the experiment is 0.15±0.05 Pa.s. After being treated with both HPP and microwave treatment, the viscosity of the honey insignificantly (P>0.05) changed regardless of the treatment and storage time. Microwave-treated honey also showed similar results, where the viscosity decreased slightly after treatment and then increased slightly during storage. However, all results are statistically insignificant (P˃0.05).
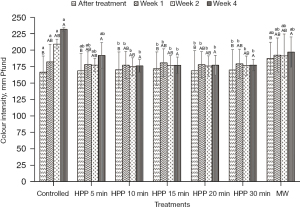
Colour intensity
As shown in Figure 5, the colour intensity of the honey exhibited minimal changes at different treatment methods and storage times. The intensity of controlled or untreated honey colour increased from Week 0 to Week 4. The colour intensity of the controlled honey increased about 39% from the original value of 167 mm Pfund. Meanwhile, HPP-treated honey retained its colour intensity and was not significantly (P˃0.05) affected under all treatment conditions. The result shows that HPP can maintain the colour attributes of honey. However, a slightly significant (P≤0.05) change was observed for microwave-treated honey, but the value is lower than the controlled honey. The colour intensity of the microwave-treated honey increased by 13% right after treatment at Week 0 and about 5% after the storage study at Week 4.
Sugar composition profile
The initial values of glucose, fructose, and maltose for the stingless bee honey used in the experiment are 28.19±4.32 g/100 g, 17.94±3.49 g/100 g, and 25.49±2.58 g/100 g, respectively (Figures 6-8). From the results, the values of the sugars at all treatment conditions showed a decrement, except for the honey sample treated for 5 min. The HPP sample treated for 5 min indicated that all simple sugars were preserved after treatment at Week 0 and at the end of the storage study (Week 4). From Table 1, all sums of glucose and fructose for all honey samples are within the permissible value of Malaysian Standard MS 2683 [2017] (11), which is not more than 90.0 g/100 g for processed honey. The highest value recorded for the sum of glucose and fructose is 46.13 g/100 g. However, the maltose level exceeded the guideline for all treatments, and the storage conditions were supposed to be lower than 10.0 g/100 g.

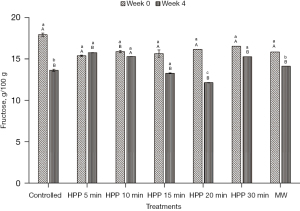
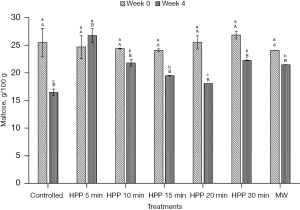
Table 1
| Storage time/treatments | Fructose + glucose (g/100 g) | F/G ratio | G/M ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 0 | Week 4 | Week 0 | Week 4 | |||
| Controlled | 46.13±3.91 | 36.56±0.16 | 0.64 | 0.59 | 1.07 | 0.83 | ||
| HPP 5 min | 41.56±0.08 | 43.66±0.51 | 0.59 | 0.56 | 1.00 | 1.04 | ||
| HPP 10 min | 42.59±0.22 | 39.47±0.08 | 0.60 | 0.63 | 1.01 | 0.91 | ||
| HPP 15 min | 41.62±0.07 | 34.04±0.03 | 0.60 | 0.64 | 0.99 | 0.77 | ||
| HPP 20 min | 43.31±0.66 | 31.27±0.02 | 0.60 | 0.63 | 1.02 | 0.72 | ||
| HPP 30 min | 44.40±0.38 | 38.59±0.09 | 0.59 | 0.65 | 1.04 | 0.88 | ||
| MW | 41.44±0.01 | 36.83±0.03 | 0.62 | 0.62 | 1.02 | 0.85 | ||
HPP, high-pressure processing; MW, microwave pasteurization; F/G, fructose/glucose ratio; G/M, glucose/moisture ratio.
Effect of HPP and microwave pasteurisation on the formation of HMF in stingless bee honey
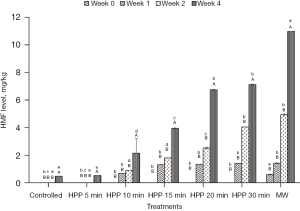
HMF level
Figure 9 shows the HMF content in controlled honey (without treatment) as well as HPP and microwave-treated honey. HMF was absent in all honey samples at Week 0 right after treatment, except microwave-treated honey. After being microwave pasteurised, the honey exhibited a low HMF level due to the heating process. As the storage time increased, the formation of HMF in honey started to increase at Week 2, except for the control, HPP-treated honey for 5 min, and MW-treated honey. The highest HMF value recorded is 12 mg/kg (microwave-treated honey), which is below the permissible value by Malaysian Standard MS 2683 [2017] (11) of 30 mg/kg and the Codex Alimentarius Commission [2001] [13] of 40 mg/kg. As for the HPP-treated honey, the HMF level also increased with treatment time.
Discussion
Effect of HPP and microwave pasteurisation on the physicochemical properties of stingless bee honey and storage stability
Moisture content
The result obtained is similar to the results reported by De La Paz Moliné et al. [2015] (44), where the moisture content of their honey decreased after being microwave treated because the honey experienced a temperature rise due to the effect of microwave radiation exposure. Hebbar et al. [2003] (23) supported the previous statement, where they found that the moisture reduction could be as high as 9% at larger power levels of microwave treatment. However, HPP treatment does not affect the moisture content of the honey. Fauzi & Farid [2016] (51) reported similar results, where the moisture content of Manuka honey changed slightly but was statistically insignificant after HPP treatment for 10 min at 600 MPa and ambient temperature. Most studies by other researchers applied HPP to foods, especially fruit juices, meat, and smoothies. The application of HPP on honey, especially stingless bee honey, is still scarce. Most of the works are about how HPP can preserve the colour and antioxidant properties of foods. Therefore, information on the effect of HPP on the physicochemical properties of honey is rarely found.
pH and free acidity
Until today, the research on the effect of HPP treatment on the pH value and free acidity of stingless bee honey is relatively scarce. In this present work, the pH value of the honey is not affected by both treatment type and storage duration on the honey. Both studies by Chen et al. [2015] (52) and Fauzi & Farid [2016] (51) supported the previous statement as no remarkable changes were observed in the pH value of asparagus juice and Manuka honey, respectively, due to the effect of HPP. Meanwhile, the free acidity of the controlled honey increased during storage duration. The controlled honey might undergo fermentation during storage. The free acidity level of the HPP and microwave-treated honey remained unaffected throughout the storage duration. This result proves that both HPP and microwave treatment can prevent the fermentation of stingless bee honey. The study of microwave-treated honeybee honey by De La Paz Moliné et al. [2015] (44) supported the statement that the acidity of the eight samples of honey in their work also remained unaffected by the microwave treatment. All honey samples in this present study show permissible pH values under the Codex Alimentarius Commission [2001] (13), where the pH of honey should be between pH 3.2 and 4.5, and for Malaysian Standard MS 2683 [2017] (11), the pH should be in the range of pH 2.5–3.8. Therefore, it can be concluded that the pH and free acidity of stingless bee honey can also be preserved through pretreatment, either HPP or microwave, before being commercialised to end consumers.
Viscosity
Viscosity is affected by the moisture level in the honey (53), and sugar composition also contributes to the rheology properties of the honey. Fructose and glucose play important roles as monosaccharides in honey as a high amount of these individual sugars may result in low viscous honey. On the contrary, the combination of these sugars with a glycosidic bond may lead to the formation of higher molecular weight compounds, namely disaccharides (maltose), and eventually increases the viscosity of honey. Honey with high sugar concentration and acidity can be the pertinent factors that promote glucose and fructose combination. However, it is proven in this study and supported by other work by Fauzi & Farid [2016] (51) that HPP can also prevent the combination of individual sugar. Therefore, the viscosity of the honey is preserved in the present study, where this is one of the critical features that affect consumer acceptance.
Colour intensity
According to De La Paz Moliné et al. [2015] (44), their work also attained similar results, where the value of the colour intensity of microwave-treated honey increased after treatment. They believed that the acceleration of the Maillard reaction is one of the main effects of thermal treatment, which affects the colour intensity of honey. Maillard reaction is derived from non-chemical browning transition, which leads to the establishment of some brown pigments and even intermediate products, such as HMF. This is supported by Starowicz et al. [2021] (54), who stated that the Maillard reaction leads to the formation of brown pigments and is strongly correlated with the antioxidant potential of honey.
Sugar composition profile
From the results, sugar level of the honey experienced a decrement except for the honey sample treated for 5 min. One of the reasons for the decrease in sugar is the conversion of sugar into HMF throughout the experiment and the storage time. Previously, Akhmazillah et al. [2013] (38), Al-Habsi & Niranjan [2012] (55), Fauzi et al. [2014] (42), Fauzi & Farid [2016, 2015] (51,56), and Razali et al. [2019] (43) studied the effect of HPP on the quality features in honey. However, no previous study has reported the effect of either HPP or microwave treatment on the sugar composition of honey, especially stingless bee honey. Liu et al. [2014] (57) studied the effect of HPP (600 MPa/1 min) on mango nectars, where the amount of fructose and glucose decreased significantly after treatment. The authors concluded that the decrease of sugars (fructose and glucose) is closely related to the Maillard reaction during different treatment methods, and this is supported by the significant increase of HMF. In contrast, Andrés et al. [2016] (58) and Butz et al. [2003] (59) stated in their studies that HPP did not affect the fructose and glucose levels of soy and milk smoothies, orange, raspberry, tomato, carrot, strawberry, apple, and peach juices. The effect of HPP on food quality, especially sugar composition, seems to be inconsistent, which requires further studies in the near future. However, the high maltose value is incoherent with most other studies on tropical-originated stingless bee honey, where the maltose value is higher than 10.0 g/100 g (19,60). Based on the fructose/glucose ratio, all honey is likely to crystallise rapidly as the value of the F/G ratio is less than 1.0 (61). Honey with less than 30% of glucose and a more significant fructose amount crystallises quite slowly and can remain liquid for many years without prior treatment. On the other hand, the authors added that as the glucose/water ratio is less than 1.7, it is an indicator that crystallisation would be very slow or null. Honey treated at 600 MPa for 5 min is the best for sugar preservation.
Effect of HPP and microwave pasteurisation on the formation of HMF in stingless bee honey
HMF level
The formation of the HMF compound in the microwave-treated honey might be due to the increase in temperature during treatment, which causes the browning process called the Maillard reaction. The HMF level is proven to be affected by high-temperature treatment and long storage time. Another study by Razali et al. [2019] (43) reported that HPP-treated stingless bee honey could preserve the antioxidant properties, natural enzymatic activity, and colour attributes, resulting in values below the range of 1.5–3.0, unnoticeable value by human eyes. However, no HMF results were reported. Studies by Biluca et al. [2014] (62) and Braghini et al. [2019] (63) highlighted that honey subjected to high-temperature treatment and short treatment time avoided the formation of HMF. No HMF was detected in the honey treated between 75 and 95 ℃ for 20–60 s in both studies. Chuttong et al. [2016] (64) studied the effect of temperature and storage time on stingless bee honey from Thailand. The honey stored at 4 ℃ for 6 and 12 months recorded the smallest change in HMF value than the honey stored at 30 and 45 ℃ for the similar storage duration. Khalil et al. [2010] (14) claimed that any type of honey is best consumed within one year of storage. The team reported that honey stored between 3 and 6 months exhibited a permissible value of HMF content (2.80–24.87 mg/kg) while honey stored for a longer period of between 12 and 24 months exhibited a high value of HMF content (128.19–1,131.76 mg/kg), exceeding the permissible value for both Malaysian Standard MS 2683 [2017] (11) and Codex Alimentarius Commission [2001] (13). The HMF content of microwave-treated honey was found to be the highest compared to HPP-treated honey at all different treatment times. This result is supported by the study of De La Paz Moliné et al. [2015] (44) as microwave-treated honey resulted in high HMF content. However, in the same study, microwave treatment showed potential in reducing aerobic mesophilic bacteria, moulds, yeasts, and P. larvae. Therefore, the authors concluded that microwave treatment could be beneficial or disadvantageous depending on the power and exposure time selection. Similar to HPP, Hebbar et al. [2003] (23) also stated that heating honey at a shorter time (15 s) and high power intensity (16 W/g) is the best for producing honey with a desirable HMF value (3.8 mg/kg) and also higher diastase activity (12.0). Kowalski [2013] (65) also mentioned that even though microwave heating resulted in high HMF formation, this method is suitable for honey as the treatment time is short.
Conclusions
Based on the results, there are no significant changes in both HPP and microwave-treated honey in terms of physicochemical properties, such as pH, free acidity, and viscosity. As for the moisture content and colour intensity of the honey, microwave-treated honey showed significant changes right after treatment at Week 0, where the moisture content decreased whereas the colour intensity and HMF level increased. This might correspond to the high-temperature exposure during the treatment. The colour intensity of the microwave-treated honey increased about 13% after treatment, and the value is lower than the controlled honey. Honey treated with HPP for 5 min showed the best results, where the honey remained unchanged in terms of sugar composition and HMF level after the treatment and storage time.
To conclude, HPP can be practised for larger scales of stingless bee honey production at a short treatment time to preserve most of the fresh honey quality. Meanwhile, microwave treatment may lead to the undesirable colour of honey. Nevertheless, both HPP and MW could not avoid HMF formation in the honey, which might prevent their application as the pretreatment methods in stingless bee honey processing. It is recommended to study the effect of HPP at a shorter treatment time (less than 5 min) on the physicochemical and microbial properties of stingless bee honey for future work.
Acknowledgments
Funding: This research was supported by the Trans-disciplinary Research Grant Scheme (TRGS/1/2016/UPM/01/5/3) under the Ministry of Higher Education Malaysia and Grant of the Prince Faisal bin Fahad Awards for Sports Research 2021 from the Ministry of Sport Saudi, Saudi Arabia for the research funding.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-13/rc
Data Sharing Statement: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-13/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-13/coif). YAY serves as an unpaid editorial board member of Longhua Chinese Medicine from July 2021 to June 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abd Jalil MA, Kasmuri AR, Hadi H. Stingless Bee Honey, the Natural Wound Healer: A Review. Skin Pharmacol Physiol 2017;30:66-75. [Crossref] [PubMed]
- Pimentel TC, Rosset M, de Sousa JMB, et al. Stingless bee honey: An overview of health benefits and main market challenges. J Food Biochem 2022;46:e13883. [Crossref] [PubMed]
- Koochak H, Seyyednejad SM, Motamedi H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran). Asian Pac J Trop Med 2010;3:180-4. [Crossref]
- Chanchao C. Antimicrobial activity by Trigona Laeviceps (stingless bee) honey from Thailand. Pak J Med Sci 2009;25:364-9.
- Molan PC. Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World 1999;80:80-92. [Crossref]
- da Cruz Ferreira R, de Souza Dias F, de Aragão Tannus C, et al. Essential and Potentially Toxic Elements from Brazilian Geopropolis Produced by the Stingless Bee Melipona quadrifasciata anthidioides Using ICP OES. Biol Trace Elem Res 2021;199:3527-39. [Crossref] [PubMed]
- Oliveira SS, Alves CN, Morte ES, et al. Determination of essential and potentially toxic elements and their estimation of bioaccessibility in honeys. Microchem J. 2019;151:104221. [Crossref]
- Santos Jr AF, Brandao GC, MC Santos J, et al. Multi-element composition, physicochemical and pollen attributes of honeys from the Paraguacu River (Bahia, Brazil) by inductively coupled plasma-optical emission spectrometry (ICP OES). An Acad Bras Cienc. 2020; 92.
- Popova M, Gerginova D, Trusheva B, et al. A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula ferruginea. Foods 2021;10:997. [Crossref] [PubMed]
- Shapla UM, Solayman M, Alam N, et al. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chem Cent J 2018;12:35. [Crossref] [PubMed]
- Malaysian Standard MS 2683. Kelulut (Stingless bee) honey-specification (MS2683:2017) 2017. Malaysia: SIRIM Berhad.
- Mouhoubi-Tafinine Z, Ouchemoukh S, Louaileche H, et al. Effect of storage on hydroxymethylfurfural (HMF) and color of some Algerian honey. Intl Food Res J 2018;25:1044-50.
- Codex Alimentarius Commission. Alinorm 41/10: Revised standard for honey Alinorm 2001;1:19-26.
- Khalil MI, Sulaiman SA, Gan SH. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem Toxicol 2010;48:2388-92. [Crossref] [PubMed]
- Bogdanov S, Martin P, Lullmann C. Harmonised methods of the International Honey Commission (IHC) 2002. Swiss Bee Research Centre, FAM, Liebefeld.
- Ij F, Ab MH, Salwani I, et al. Physicochemical characteristics of Malaysian stingless bee honey from Trigona Species Int Med J Malays 2017;17:187-91.
- Biluca FC, Braghini F, Gonzaga LV, et al. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J Food Compos Anals 2016;50:61-9. [Crossref]
- De Sousa JMB, de Souza EL, Marques G, et al. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT - Food Sci Technol 2016;65:645-51. [Crossref]
- Chuttong B, Chanbang Y, Sringarm K, et al. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem 2016;192:149-55. [Crossref] [PubMed]
- Suntiparapop K, Prapaipong P, Chantawannakul P. Chemical and biological properties of honey from Thai stingless bee (Tetragonula leaviceps). J Apic Res 2012;51:45-52. [Crossref]
- Cozmuta AM, Cozmuta LM, Varga C, et al. Effect of thermal processing on quality of polyfloral honey. Romanian J Food Sci 2011;1:45-52.
- Turhan I, Tetik N, Karhan M, et al. Quality of honeys influenced by thermal treatment. LWT - Food Sci Technol 2008;41:1369-99. [Crossref]
- Hebbar HU, Nandini KE, Lakshmi MC. Microwave and infrared heat processing of honey and its quality. Food Sci Technol Res 2003;9:49-53. [Crossref]
- Subramanian R, Umesh Hebbar H, Rastogi NK. Processing of honey: a review. Int J Food Prop 2007;10:127-43. [Crossref]
- Muntean MV, Marian O, Barbieru V, et al. High pressure processing in food industry – characteristics and applications. Agric Agric Sci Procedia 2016;10:377-83. [Crossref]
- Balasubramaniam VM, Farkas D. High-pressure Food Processing. Food Sci Technol Int 2008;14:413-8. [Crossref]
- Marszałek K, Mitek M, Skąpska S. The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Inno Food Sci Emerg Technol 2015;27:48-56. [Crossref]
- Patras A, Brunton NP, Da Pieve S, Butler F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Inno Food Sci Emerg Technol 2009;10:308-13. [Crossref]
- Patras A, Brunton N, Da Pieve S, et al. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Inno Food Sci Emerg Technol 2009;10:16-22. [Crossref]
- Aymerich MT, Monfort JM, Hugas M. Microbial inactivation after high-pressure processing at 600 MPa in commercial meat products over its shelf life. Inno Food Sci Emerg Technol 2004;5:451-7. [Crossref]
- Carlez A, Veciana-nogues T, Cheftel JC. Changes in colour and myoglobin of minced beef meat due to high pressure processing. LWT-Food Sci Technol 1995;28:528-38. [Crossref]
- Campus M. High pressure processing of meat, meat products and seafood. Food Eng Revs 2010;2:256-73. [Crossref]
- Kural AG, Shearer AEH, Kingsley DH, et al. Conditions for high-pressure inactivation of Vibrio parahaemolyticus in oysters. Int J Food Microbiol 2008;127:1-5. [Crossref] [PubMed]
- Kingsley DH. High pressure processing of bivalve shellfish and HPP’s use as a virus intervention Foods 2014;3:336-50. [Crossref] [PubMed]
- Fidalgo LG, Saraiva JA, Aubourg SP, et al. Effect of high-pressure pre-treatments on enzymatic activities of Atlantic mackerel (Scomber scombrus) during frozen storage. Inno Food Sci Emerg Technol 2014;23:18-24. [Crossref]
- Ramirez-suarez JC, Morrissey MT. Effect of high pressure processing (HPP) on shelf life of albacore tuna (Thunnus alalunga) minced muscle. Inn Food Sci Emerg Technol 2006;7:19-27. [Crossref]
- Chaikham P, Prangthip P. Alteration of antioxidative properties of longan flower-honey after high pressure, ultra-sonic and thermal processing. Food Biosci 2015;10:1-7. [Crossref]
- Akhmazillah MFN, Farid MM, Silva FVM. High pressure processing (HPP) of honey for the improvement of nutritional value. Inno Food Sci Emerg Technol 2013;20:59-63. [Crossref]
- Cao X, Zhang Y, Zhang F, et al. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J Sci Food Agric 2011;91:877-85. [Crossref] [PubMed]
- Keenan DF, Brunton NP, Gormley TR, et al. Effect of thermal and high hydrostatic pressure processing on antioxidant activity and colour of fruit smoothies. Inno Food Sci Emerg Technol 2010;11: -6.
- Yaldagard M, Mortazavi SA, Tabatabaie F. The principles of ultra high pressure technology and its application in food processing/preservation : A review of microbiological and quality aspects. Afr J of Biotechnol 2008;7:2739-67.
- Fauzi NA, Farid MM, Silva FVM. High-pressure processing of manuka honey: improvement of antioxidant activity, preservation of colour and flow behaviour. Food Bioprocess Technol 2014;7:2299-307. [Crossref]
- Razali MF, Fauzi NAM, Sulaiman A, et al. Effect of high-pressure processing (HPP) on antioxidant, diastase activity and colour for kelulut (stingless bee) honey. J Teknol 2019;81:91-8. [Crossref]
- De La Paz Moliné M, Fernández NJ, Medici SK, et al. Effect of microwave treatment on microbial contamination of honeys and on their physicochemical and thermal properties. Polish J of Food Nutr Sci 2015;65:119-26. [Crossref]
- Ghazali HM, Ming TC, Hashim DM. Effect on microwave heating on the storage and properties of starfruit honey. ASEAN Food J 1994;9:30-6.
- Baroyi SAHM, Yusof YA, Ghazali HM, et al. A novel method based on passive diffusion that reduces the moisture content of stingless bee (Heterotrigona itama) honey. J Food Process Eng 2019;42:13221. [Crossref]
- Mohamad Ghazali NS, Yusof YA, Ghazali HM, et al. Effect of surface area of clay pots on physicochemical and microbiological properties of stingless bee (Geniotrigona thoracica) honey. Food Biosci 2021;40:1000839. [Crossref]
- Sesta G, Lusco L. Refractometric determination of water content in royal jelly. Apidologie 2008;39:225-32. [Crossref]
- Chong KY, Chin NL, Yusof YA. Thermosonication and optimization of stingless bee honey processing. Food Sci Technol Int 2017;23:608-22. [Crossref] [PubMed]
- Silvano MF, Varela MS, Palacio MA, et al. Physicochemical parameters and sensory properties of honeys from Buenos Aires region. Food Chem 2014;152:500-7. [Crossref] [PubMed]
- Fauzi NA, Farid MM. High pressure processed manuka honey: change in nutritional and rheological properties over 1-year storage. J Food Process Pres 2016;41:e13085. [Crossref]
- Chen X, Qin W, Ma L, et al. Effect of high pressure processing and thermal treatment on physicochemical parameters, antioxidant activity and volatile compounds of green asparagus juice. LWT - Food Sci and Technol 2015;62:927-33. [Crossref]
- Bhandari B, D’Arcy B, Chow S. Rheology of selected Australian honeys. J Food Eng 1999;41:65-8. [Crossref]
- Starowicz M, Ostaszyk A, Zieliński H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021;10:967. [Crossref] [PubMed]
- Al-Habsi NA, Niranjan K. Effect of high hydrostatic pressure on antimicrobial activity and quality of Manuka honey. Food Chem 2012;135:1448-54. [Crossref] [PubMed]
- Fauzi NA, Farid MM. Original article High-pressure processing of Manuka honey: brown pigment formation, improvement of antibacterial activity and hydroxymethylfurfural content. Int J Food Sci & Technol 2015;50:178-85. [Crossref]
- Liu F, Li R, Wang Y, et al. Effects of high hydrostatic pressure and high-temperature short-time on mango nectars: Changes in microorganisms, acid invertase, 5-hydroxymethylfurfural, sugars, viscosity, and cloud. Inno Food Sci Emerg Technol 2014;22:22-30. [Crossref]
- Andrés V, Villanueva MJ, Tenorio MD. The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chem 2016;192:328-35. [Crossref] [PubMed]
- Butz P, Fern A, Lindauer R, et al. Influence of ultra high pressure processing on fruit and vegetable products. J Food Eng 2003;56:233-6. [Crossref]
- Tuksitha L, Chen YLS, Chen YL, et al. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J Asia-Pac Entomol 2018;21:563-70. [Crossref]
- Dobre I, Georgescu LA, Alexe P, et al. Rheological behavior of different honey types from Romania. Food Res Int 2012;49:126-32. [Crossref]
- Biluca FC, Della Betta F, de Oliveira GP, et al. 5-HMF and carbohydrates content in stingless bee honey by CE before and after thermal treatment. Food Chem 2014;159:244-9. [Crossref] [PubMed]
- Braghini F, Biluca FC, Gonzaga LV, et al. Impact of short-term thermal treatment on stingless bee honey (Meliponinae): Quality, phenolic compounds and antioxidant capacity. J Food Process Pres 2019;43:e13954. [Crossref]
- Chuttong B, Chanbang Y, Sringarm K, et al. Effects of long term storage on stingless bee (Hymenoptera: Apidae: Meliponini) honey. J Apic Res 2016;54:441-51. [Crossref]
- Kowalski S. Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. Food Chem 2013;141:1378-82. [Crossref] [PubMed]

