A narrative review of phytochemical profile of Saffron and principal crocetin glycosides
Introduction
Crocus sativus L. (Iridaceae), well-known native to the eastern Mediterranean-Europe and western Asia region, is a perennial stemless herb that is widely cultivated in many temperate countries including Spain, Greece, Turkey, Iran, India, China, and Japan (1,2) (Figure 1). The massive cultivation of C. sativus is timely developed to produce such the well-known and costly spice “red gold”, called saffron as stigmas of its flower. Since saffron has unique color, aroma, and taste, it is widely used as a spice, coloring and flavoring agent in food and cosmetic products. In addition, it displays a variety of health benefits and has been used in both traditional and modern medicines (3,4).

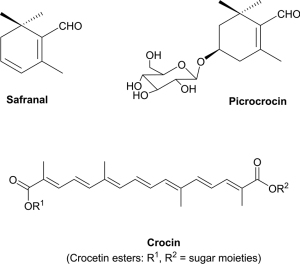
Phytochemical components of saffron have been extensively reported (5-8), in which safranal, picrocrocin, and crocetin glycosides (crocin) are major components and responsible for typical bitter taste, spicy aroma, and red color, respectively (Figure 2). Crocetin glycosides such as crocins 1–4 are dominated non-volatile components and be marker compounds for quality control and standardization of saffron (5). In addition, the other aspects of natural product chemistry of the crocin such as chemical structure, biosynthesis, and biodiversity have been investigated and reported (6-8). This review then will be focused on the crocetin glycosides as the principal composition of the title material under the viewpoint of natural product chemistry.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-70/rc).
Methods
This narrative review consists of a PubMed search of English publication up to date of January 2022. The keywords used for the search were consecutively: “saffron and crocin OR Crocus and crocin”. This first step released 227 articles. Then, manual screening of the 11 words cited above in Table 1 referring to saffron and crocin were realized for the 227 articles. Sixty articles were ultimately used, as listed in the references.
Table 1
| Items | Specification |
|---|---|
| Date of search | October 2021 |
| Database and other sources searched | PubMed |
| Search term used | Search terms: saffron and crocin OR Crocus and crocin |
| Timeframe | Before October 2021 |
| Inclusion criteria | Inclusion criteria: |
| (I) Article languages: English | |
| (II) Article types: all | |
| (III) Articles only relates to saffron or crocetin structure (manual screening of the following words in the abstracts or the whole articles: saffron, Crocus sativus, crocin, crocetin, safranal, picrocrocin, structure, NMR, spectroscopic data, analysis, biosynthesis, pharmacological activity | |
| Selection process | NH Tung, NN Hieu, and VV Tuan conducted the selection together |
Phytochemical profile of saffron
Principal components
Since first phytochemical report on saffron by Zarghami in 1971 (6), together with development and advancement of chromatography, the phytochemical profile of saffron has been very attractive to Worldwide natural product chemists and being studied in detail and, to date, reveal the occurrence of totally more than 150 volatile and non-volatile compounds (7). In which, there are about 60 constituents have been identified by conventional isolation and structural elucidation. The volatiles consist of more than 40 components that are mainly monoterpenes, their alcohol and ester derivatives, of which, safranal (2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde, 60-70% of the volatiles) is the main component. Non-volatile components include crocetin, crocin, picrocrocin and flavonoid. The detailed all the compounds from saffron is listed in the interesting review article by Mykhailenko et al. (8).
Being well documented and notably, crocin is the unique water water-soluble carotenoids found in the title material and the primary component responsible for the red color of saffron. Chemically, crocin is a series of compounds that are glycosyl esters of crocetin are the major components of saffron (9,10). The quantitative study revealed the main crocins including crocin-1, crocin-2, crocin-3 and crocin-4 as shown in Table 2 and Figure 3 (12-15). The detailed outline and insight discussion on their structure, spectroscopic data, distribution, and so on will be addressed mainly in the main following section.
Table 2
| Compounds | Chemical name | Molecular formula, molecule weight | Weight content (%, wt/wt) | Reference |
|---|---|---|---|---|
| Safranal | 2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde | C10H14O, 150 | 0.24/1.0 | (5,7,8) |
| Picrocrocin | 4-(β-D-glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1-carbaldehyde | C16H26O7, 330 | 7.0/16.0 | (5,7,8) |
| Crocin (crocin 1–4) | Crocetin esters e.g., crocin 1–4: Crocetin di-(β-D-gentiobiosyl) ester | C44H64O24, 976 | 16.0/30.0; 6.4/18.0 (crocin–4) | (5,7,8) |
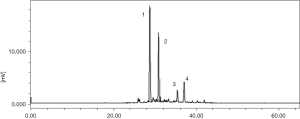
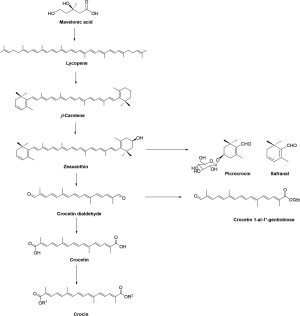
Picrocrocin is glycosylated form of safranal and chemically different from crocin but in the viewpoint of biosynthesis, the biosynthesis pathways of three principal components of saffron have been established and relatively from the same precursor of zeaxanthin (16,17). Figure 4 showed the brief biosynthesis pathway with the key intermediate and precursor (18).
The profile of crocin, picrocrocin and safranal contributes to quality control of saffron (19,20) and it has been suggested that the best quality saffron should contain about more than 20% crocin, 5% picrocrocin, and 0.5% safranal (21).
Crocetin and its glycosides
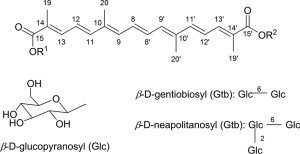
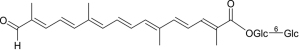
Crocins are crocetin glycosides, in which structure variation depends on the sapogenin backbone of crocetin (C20H24O4; MW: 328.4 g/mol) and, mainly, the terminal sugar chains (glucose, gentibiose, and neapolitanose) (8). Crocetin framework inheriting from zeaxanthin in the proven biosynthesis pathway with a long chain of conjugated carbon-carbon (seven conjugated double bond and two terminal carbonyl function, 4 side-chain methyl groups) is relatively unstable with temperature, oxidants, UV radiation, and pH condition resulting fragmentation of certain double bond of polyene backbone other than variable functional groups. The long-conjugated system of the crocetin backbone makes the crocins appear in red color and can be detected by UV-VIS spectra (characteristic maxima VIS absorbance in the range 400–500 nm), which are helpful in both qualitative and quantitative analyses. In addition, the stereochemistry of crocetin and crocins is only from conformation of double bond and highly oriented in one form, whether cis or trans and, normally, the trans-form is more stable than the cis-form. The cis/trans conformation make influence on their conjugated systems and results in their different UV-VIS spectra at secondary peak beside the characteristic peaks, the cis isomer gives a single peak between 320 and 340 nm and, in case of all-trans isomer, the secondary peak is at ca 256 nm. As shown in Table 3 and Figure 5, since first crocins were isolated in 1975 (30) and then structurally identified in 1982 (24), up to date, there have been 22 compounds including crocetin and crocin derivatives reported from saffron from noteworthy investigation of Speranza in 1984 (31), Tarantilis in 1995 (21), Carmona in 2006 (26), Dufresne in 1999 (11), Shoyama in 2013 (32), and Llorens in 2015 (12), respectively. Among them, four main crocin pigments (crocin-1, crocin-2, crocin-3, and cocin-4), named according to the number of sugar unit in their respective molecules along with minor components of α,β-carotenes, lycopene, zeaxanthin, phytoene, phytofluene, (4R)-4-hydroxy-3,5,5-trimethylcyclohex-2-enone 4-O-β-D-glucopyranoside, (4S)-4-hydroxy-3,5,5-trimethylcyclohex-2-enone 4-O-β-D-glucopyranoside, (4S)-4-(hydroxymethyl)-3,5,5-trimethylcyclohex-2-enone 4-O-β-D-glucopyranoside, and β-D-gentiobiosyl ester of 2-methyl-6-oxohepta-2,4-dienoic acid, respectively (30,33). In our previous study on saffron by dark cultivation in Japan, it is noteworthy that a unique minor crocetin glycoside was isolated and identified as trans-crocetin 1-al 1ʹ-O-β-gentiobiosyl ester based on the extensive chemical and spectroscopic evidence (Figure 6) (33). Table 4 summarizes the NMR data of the typical crocins from the original research (32-35) for forthcoming references. In term of the NMR spectroscopic data, since the structure of crocetin and the crocins are highly symmetric, the NMR signals appears whether in couples or integrated peaks. The 13C NMR spectra display even number of peaks at the range of ẟ 120–150 ppm for olefinic double bonds and, especially, two downfield signals at ẟ 165–190 ppm accounting for two terminal carbonyl carbons. It becomes evident that the relative downfield shift of the carbonyl carbon discriminates the carbonyl functions (ester, carboxylic acid, aldehyde) (32-35).
Table 3
| No. | Chemical structure | Chemical name | Molecular formula, molecule weight (amu) | Configuration/isomer (cis/trans) | References |
|---|---|---|---|---|---|
| 1 | R1 = R2 = H | 2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioic acid (Crocetin) | C20H24O4, 328 | Cis/trans | (22,23) |
| 2 | R1 = H | Crocetin 1-β-D-glucopyranosyl ester (crocin-1) | C26H34O9, 490 | Cis/trans | (21,23) |
| R2 = Glc | |||||
| 3 | R1 = H | Crocetin 1-β-D-gentiobiosyl ester (crocin-2) | C32H44O14, 652 | Cis/trans | (21,24) |
| R2 = Gtb | |||||
| 4 | R1 = Glc | Crocetin 1-β-D-glucopyranosyl 1ʹ-β-D-gentiobiosyl ester (crocin-3) | C38H54O19, 814 | Cis/trans | (21,25,26) |
| R2 = Gtb | |||||
| 5 | R1 = Gtb | Crocetin 1,1ʹ-di-β-D-gentiobiosyl ester (crocin-4) | C44H64O24, 976 | Cis/trans | (21,25,26) |
| R2 = Gtb | |||||
| 6 | R1 = Me | Crocetin β-D-glucopyranosylmethyl ester | C27H36O9, 504 | Trans | (27,28) |
| R2 = Glc | |||||
| 7 | R1 = Me | Crocetin dimethyl ester | C22H28O4, 356 | Trans | (23,28) |
| R2 = Me | |||||
| 8 | R1 = Gtb(Ac)7 | Crocetin di-(2,3,4,8,9,10,12-hepta-O-acetyl-β-D-gentiobiosyl)-ester | C72H92O38, 1,424 | Trans | (24) |
| R2 = Gtb(Ac)7 | |||||
| 9 | R1 = Glc(Ac)4 | Crocetin di-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl) ester | C60H80O32, 1,232 | Trans | (24) |
| R2 = Glc(Ac)4 | |||||
| 10 | R1 = Glc(6-1)Glc(6-1)Glc | Crocetin-(tri-β-D-glucopyranosyl)-(β-D-gentiobiosyl) ester | C50H74O29, 1,138 | Cis/trans | (26,27) |
| R2 = Gtb | |||||
| 11 | R1 = Glc | Crocetin di-(β-D-glucopyranosyl) ester | C32H44O14, 652 | Cis/trans | (21,24,26) |
| R2 = Glc | |||||
| 12 | R1 = Npt | Crocetin (β-D-neapolitanosyl)-(β-D-gentiobiosyl) ester | C50H74O29, 1,138 | Cis/trans | (21,26,29) |
| R2 = Gtb | |||||
| 13 | R1 = Npt | Crocetin (β-D-neapolitanosyl)-(β-D-glucopyranosyl) ester | C44H64O24, 976 | Cis/trans | (26) |
| R2 = Glc |
Table 4
| Position | Crocin-1 | Crocin-2 | Crocin-3 | Crocin-4 | Crocetin 1-al 1ʹ-O-β-gentiobiosyl ester |
|---|---|---|---|---|---|
| Crocetin moiety | |||||
| 8 | 166.7 | 166.6 | 166.7 | 166.7 | 168.6 |
| 9 | 127.5 | 127.5 | 125.8 | 125.6 | 126.1 |
| 10 | 140.3 | 140.3 | 140.4 | 140.4 | 142.0 |
| 11 | 124.7 | 124.6 | 124.4 | 124.3 | 124.3 |
| 12 | 145.0 | 145.0 | 145.1 | 145.0 | 146.6 |
| 13 | 137.4 | 137.3 | 137.4 | 137.4 | 139.8 |
| 14 | 136.5 | 136.4 | 136.5 | 136.5 | 139.3 |
| 15 | 132.5 | 132.5 | 132.5 | 132.5 | 135.7 |
| 19 | 13.0 | 12.9 | 13.2 | 13.1 | 13.0 |
| 20 | 13.0 | 12.9 | 13.1 | 13.0 | 12.8 |
| 8ʹ | 169.6 | 169.5 | 166.7 | 166.7 | 189.6 |
| 9ʹ | 125.7 | 125.6 | 125.8 | 125.6 | 141.9 |
| 10ʹ | 138.5 | 138.5 | 140.4 | 140.4 | 148.7 |
| 11ʹ | 124.2 | 124.2 | 124.4 | 124.3 | 123.4 |
| 12ʹ | 143.7 | 143.7 | 145.1 | 145.0 | 145.8 |
| 13ʹ | 137.1 | 137.1 | 137.4 | 137.4 | 137.7 |
| 14ʹ | 135.7 | 135.7 | 136.5 | 136.5 | 137.1 |
| 15ʹ | 132.0 | 132.0 | 132.5 | 132.5 | 135.3 |
| 19ʹ | 13.3 | 13.2 | 13.2 | 13.1 | 12.7 |
| 20ʹ | 13.1 | 13.0 | 13.1 | 13.0 | 14.5 |
| Sugar moiety | |||||
| 1ʺ | 95.0 | 95.0 | 95.1 | 95.0 | 96.0 |
| 2ʺ | 73.0 | 72.9 | 72.9 | 72.9 | 75.1 |
| 3ʺ | 76.9 | 76.7 | 77.4 | 77.3 | 77.9 |
| 4ʺ | 70.0 | 69.7 | 69.7 | 69.7 | 71.0 |
| 5ʺ | 78.3 | 78.3 | 78.3, 77.3 | 77.2 | 78.0 |
| 6ʺ | 61.0 | 68.4 | 68.4, 61.0 | 68.2 | 69.5 |
| 1ʹʺ | – | 103.5 | 103.6 | 103.5 | 104.6 |
| 2ʹʺ | – | 73.9 | 73.9 | 73.9 | 74.0 |
| 3ʹʺ | – | 77.2 | 77.4 | 77.3 | 77.9 |
| 4ʹʺ | – | 70.4 | 70.0 | 70.4 | 71.5 |
| 5ʹʺ | – | 77.3 | 76.8 | 76.7 | 78.8 |
| 6ʹʺ | – | 61.4 | 61.4 | 61.4 | 62.7 |
Quantitatively, standard saffron contain total crocin content not less than 16% but some saffron were reported to be more than 20% crocins with crocin-4 up to 16% (36,37). Furthermore, the chemical fingerprint of individual crocins in saffron have been studied by various colorimetric (38,39) and chromatography techniques (HPTLC, HPLC, UPLC…) (40-44), especially modern hyphenated, GC-MS, FTIR, LC-MS and LC-MS-NMR methods (45-48), so that minor compounds and trace components have been identified. Recently, Aiello and co-workers reported a LC-MALDI MS method for fingerprinting and quantitative analysis of saffron and revealed occurrence of potential and not reported crocetin esters with long sugar chains of four-to-seven sugar units, molecular weight up to 1800 amu in advance (49). These findings are consistent with the biosynthesis pathway of crocin and support variation in biosynthesis as well as potential of new crocin derivative in saffron and Crocus genus.
Under viewpoint of chemotaxonomy, accumulating data shows that crocetin and its glycosides have been occurred in very few medicinal plants including Crocus genus of Iridaceae [C. sativus, C. neapolitanus (50), C. speciosus, C. luteus (51)], Gardenia jasminoides (Rubiaceae) (34,35), Arctium lappa (Asteraceae) (52,53), Mimosa pudica (Leguminosae) (54), Buddleja officinalis (Loganiaceae) (55), Stemona japonica (Stemonaceae) (56), Nyctanthes arbor-tristis (Oleaceae) (57), Jacquinia angustifolia (58), Coleus forskolii (59), and Artocarpus heterophyllus (60), respectively. Of which, the saffron and the fruit of G. jasminoides are most dominated in contents of crocetin and various crocetin glycosides. In the title herb of C. sativus, crocetin and crocetin glycosides are only found in the saffron. So that, crocetin and certain its glycosides contribute impact meaning in chemotaxonomy of Crocus species and others.
Conclusions
Saffron is the well-known traditional medicinal herb and potential application in the modern medicine based on its scientific database, especially the phytochemical profile. This brief review as updating due time and, once again, further supports that crocin is the principal content and defined the saffron quality. In addition, the potential of new crocin have been evidenced by chromatographic fingerprint and need to be more studied.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Longhua Chinese Medicine for the series “Multifunctional Saffron”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-70/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-70/coif). The series “Multifunctional Saffron” was commissioned by the editorial office without any funding or sponsorship. YS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mousavi SZ, Bathaie SZ. Historical uses of saffron: identifying potential new avenues for modern research. Avicenna J Phytomed 2011;1:57-66.
- Basker D, Negbi M. Uses of saffron. Econ Bot 1983;37:228-36. [Crossref]
- Srivastava R, Ahmed H, Dixit RK, et al. Crocus sativus L.: A comprehensive review. Pharmacogn Rev 2010;4:200-8. [Crossref] [PubMed]
- Morimoto S, Umezaki Y, Shoyama Y, et al. Post-harvest degradation of carotenoid glucose esters in saffron. Planta Med 1994;60:438-40. [Crossref] [PubMed]
- Ríos JL, Recio MC, Giner RM, et al. An update review of saffron and its active constituents. Phytother Res 1996;10:189-93. [Crossref]
- Zarghami NS, Heinz DE. Monoterpene aldehydes and isophorone-related compounds of saffron. Phytochemistry 1971;10:2755-61. [Crossref]
- Liakopoulou-Kyriakides M, Kyriakidis D. Crocus sativus-biological active constituents. Stud Nat Prod Chem 2002;16:293-312. [Crossref]
- Mykhailenko O, Kovalyov V, Goryacha O, et al. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019;162:56-89. [Crossref] [PubMed]
- Tong Y, Zhu X, Yan Y, et al. The influence of different drying methods on constituents and antioxidant activity of saffron from china. Int J Anal Chem 2015;2015:953164. [Crossref] [PubMed]
- Gregory MJ, Menary RC, Davies NW. Effect of drying temperature and air flow on the production and retention of secondary metabolites in saffron. J Agric Food Chem 2005;53:5969-75. [Crossref] [PubMed]
- Dufresne C, Cormier F, Dorion S, et al. Glycosylation of encapsulated crocetin by a Crocus sativus L. cell culture. Enzym Microb Technol 1999;24:453-62. [Crossref]
- Llorens S, Mancini A, Serrano-Díaz J, et al. Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension. Molecules 2015;20:17570-84. [Crossref] [PubMed]
- Ahrazem O, Trapero A, Gómez MD, et al. Genomic analysis and gene structure of the plant carotenoid dioxygenase 4 family: a deeper study in Crocus sativus and its allies. Genomics 2010;96:239-50. [Crossref] [PubMed]
- Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem 2007;100:1126-31. [Crossref]
- Maggi L, Carmona M, Zalacain A, et al. Changes in saffron volatile profile according to its storage time. Food Res Int 2010;43:1329-34. [Crossref]
- Liu T, Yu S, Xu Z, et al. Prospects and progress on crocin biosynthetic pathway and metabolic engineering. Comput Struct Biotechnol J 2020;18:3278-86. [Crossref] [PubMed]
- Mir JI, Ahmed N, Wafai AH, et al. Relative expression of CsZCD gene and apocarotenoid biosynthesis during stigma development in Crocus sativus L. Physiol Mol Biol Plants 2012;18:371-5. [Crossref] [PubMed]
- Lozano P, Delgado D, Gomez D, et al. A nondestructive method to determine the safranal content of saffron (Crocus sativus L.) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography. J Biochem Biophys Methods 2000;43:367-78. [Crossref] [PubMed]
- Kanakis CD, Daferera DJ, Tarantilis PA, et al. Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde (HTCC) in Greek saffron. J Agric Food Chem 2004;52:4515-21. [Crossref] [PubMed]
- Tarantilis PA, Polissiou M, Manfait M. Separation of picrocrocin, cis-trans crocins and safranal of saffron using high-performance liquid chromatography with photodiode-array detection. J Chromatogr A 1994;664:55-61. [Crossref] [PubMed]
- Tarantilis PA, Tsoupras G, Polissiou M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J Chromatogr A 1995;699:107-18. [Crossref] [PubMed]
- Tarantilis PA, Polissiou MG. Isolation and identification of the aroma components from saffron (Crocus sativus). J Agric Food Chem 1997;45:459-62. [Crossref]
- García-Rodríguez MV, López-Córcoles H, Alonso GL, et al. Comparative evaluation of an ISO 3632 method and an HPLC-DAD method for safranal quantity determination in saffron. Food Chem 2017;221:838-43. [Crossref] [PubMed]
- Pfander H, Schurtenberger H. Biosynthesis of C20-carotenoids in Crocus sativus L. Phytochemistry 1982;21:1039-42. [Crossref]
- Straubinger M, Bau B, Eckstein S, et al. Identification of novel glycosidic aroma precursors in saffron (Crocus sativus L.). J Agric Food Chem 1998;46:3238-43. [Crossref]
- Carmona M, Zalacain A, Sánchez AM, et al. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J Agric Food Chem 2006;54:973-9. [Crossref] [PubMed]
- Zhou J, Xie G, Yan X. Encyclopedia of Traditional Chinese Medicines–Molecular, Structures, Pharmacological Activities, Natural Sources and Applications. Isolated Compounds A-C, vol. 1. Springer-Verlag, Berlin, 2011:3934.
- Moraga AR, Rambla JL, Ahrazem O, et al. Metabolite and target transcript analyses during Crocus sativus stigma development. Phytochemistry 2009;70:1009-16. [Crossref] [PubMed]
- Pfister S, Meyer P, Steck A, et al. Isolation and structure elucidation of carotenoid glycosyl esters in Gardenia fruits (Gardenia jasminoides) and saffron (Crocus sativus Linn.). J Agric Food Chem 1996;44:2119-22. [Crossref]
- Pfander H, Wittwer F. Carotenoid-glycosides. Investigation of carotenoid-composition of saffron. Helv Chim Acta 1975;58:1608-20. [Crossref] [PubMed]
- Speranza G, Dada G, Manitto P, et al. 13-Cis crocin: a new crocinoid of saffron. Gazz Chim Ital 1994;114:189-92.
- Tung NH, Shoyama Y. New minor glycoside components from saffron. J Nat Med 2013;67:672-6. [Crossref] [PubMed]
- Straubinger M, Jezussek M, Waibel R, et al. Novel Glycosidic Constituents from Saffron. J Agric Food Chem 1997;45:1678-81. [Crossref]
- Choi HJ, Park YS, Kim MG, et al. Isolation and characterization of the major colorant in Gardenia fruit. Dyes and Pigments 2001;49:15-20. [Crossref]
- Wang Y, Chen Y, Deng L, et al. Systematic separation and purification of iridoid glycosides and crocetin derivatives from Gardenia jasminoides Ellis by high-speed-counter-current chromatography. Phytochem Anal 2015;26:202-8. [Crossref] [PubMed]
- Kyriakoudi A, Chrysanthou A, Mantzouridou F, et al. Revisiting extraction of bioactive apocarotenoids from Crocus sativus L. dry stigmas (saffron). Anal Chim Acta 2012;755:77-85. [Crossref] [PubMed]
- Ordoudi SA, Tsimidou MZ. Saffron quality: effect of agricultural practices, processing and storage. In: Dris R, Jain SM (eds.). Production Practices and Quality Assessment of Food Crops. Netherlands: Springer Dordrecht, 2004.
- Orfanou O, Tsimidou M. Evaluation of the colouring strength of saffron spice by UV—Vis spectrometry. Food Chem 1996;57:463-9. [Crossref]
- Alonso GL, Sanchez MA, Salinas MR, et al. Saffron color analysis. Alimentoria (Madrid) 1997;279:115-27.
- Iborra J, Luis C, Rosario M, et al. TLC Preparative Purification of Picrocrocin, HTCC and Crocin from Saffron. J Food Sci 1992;57:714-16. [Crossref]
- Sujata V, Ravishankar GA, Venkataraman LV. Methods for the analysis of the saffron metabolites crocin, crocetins, picrocrocin and safranal for the determination of the quality of the spice using thin-layer chromatography, high-performance liquid chromatography and gas chromatography. J Chromatogr A 1992;624:497-502. [Crossref]
- Lozano P, Simancas MJ, Iberia JL. Quantitative high-performance liquid chromatographic method to analyze commercial saffron (Crocus Sativus L.) products. J Chromatogr A 1999;830:477-83. [Crossref]
- Li N, Lin G, Kwan YW, et al. Simultaneous quantification of five major biologically active ingredients of saffron by high-performance liquid chromatography. J Chromatogr A 1999;849:349-55. [Crossref] [PubMed]
- Anastasaki E, Kanakis C, Pappas C, et al. Geographical differentiation of saffron by GC–MS/FID and chemometrics. Eur Food Res Technol 2009;229:899-905. [Crossref]
- Xu S, Ge X, Li S, et al. Discrimination of Different Parts of Saffron by Metabolomic-Based Ultra-Performance Liquid Chromatography Coupled with High-Definition Mass Spectrometry. Chem Biodivers 2019;16:e1900363. [Crossref] [PubMed]
- Petrakis EA, Polissiou MG. Assessing saffron (Crocus sativus L.) adulteration with plant-derived adulterants by diffuse reflectance infrared Fourier transform spectroscopy coupled with chemometrics. Talanta 2017;162:558-66. [Crossref] [PubMed]
- Petrakis EA, Cagliani LR, Polissiou MG, et al. Evaluation of saffron (Crocus sativus L.) adulteration with plant adulterants by (1)H NMR metabolite fingerprinting. Food Chem 2015;173:890-6. [Crossref] [PubMed]
- Yilmaz A, Nyberg NT, Molgaard P, et al. H-1 NMR metabolic fingerprinting of saffron extracts. Metabolomics 2010;6:511-7. [Crossref]
- Aiello D, Siciliano C, Mazzotti F, et al. Molecular species fingerprinting and qualitative analysis of saffron (Crocus sativus L.) for quality control by MALDI mass spectrometry. RSC Advances 2018;8:36104-13. [Crossref] [PubMed]
- Rychener M, Bigler P, Pfander H. Isolierung und strukturaufklärung von neapolitanose (O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→6)]-(D-glucose), einem neuen trisaccharid aus den stempeln von gartenkrokussen (Crocus neapolitanus var.). Helv Chem Acta 1984;67:386-91. [Crossref]
- Harborne JB, Williams CA. 6-Hydroxyflavones and other flavonoids of Crocus. Z. Naturforsch 1984;39c:18-23. [Crossref]
- Tang Y, Lou Z, Yang L, et al. Screening of Antimicrobial Compounds against Salmonellaty Phimurium from Burdock (Arctium lappa) Leaf Based on Metabolomics. Eur Food Res Technol 2015;240:1203-9. [Crossref]
- Wang D, Bădărau AS, Swamy MK, et al. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front Plant Sci 2019;10:834. [Crossref] [PubMed]
- Patel NK, Bhutani KK. Suppressive effects of Mimosa pudica (L.) constituents on the production of LPS-induced pro-inflammatory mediators. EXCLI J 2014;13:1011-21. [PubMed]
- Shi L, Xie GY, Wang S, et al. Advance in Pharmaceutical Research of Buddleia officinalis Maxim. Chin Wild Plant Resour 2016;35:34-40.
- Yang XZ, Tang CP. Chemical Constituents of Stemona japonica. Nat Product Res Development 2008;020:399-402.
- Pawar DN, Panchal SS, Kumaravelu J, et al. Crocin Rich Extract of Nyctanthes arbor-tristis Flower Calyx Induces Anti-angiogenic Activity. Nat Prod J 2015;6:40-8. [Crossref]
- Eugster CH, Hurlimann H, Leuenberger HJ. Crocetindialdehyd und crocetinhalbaldehyd als blutenfarbstoffe von Jacquinia angustifolia. Helv Chim Acta 1969;52:806-7. [Crossref]
- Tandon J, Katti S, Ruedi P, et al. Crocetin-dialdehyde from Coleus forskohlii. Helv Chim Acta 1979;62:2706-7. [Crossref]
- Priyadarshani AMB, Jansz E, Peiris H. Studies on the carotenoids of jakfruit (Artocarpus heterophyllus Lam.) from Matale and Kurunegala districts. J Natl Sci Found Sri Lanka 2007;35:259-62. [Crossref]
Cite this article as: Tung NH, Hieu NN, Tuan VV, Shoyama Y. A narrative review of phytochemical profile of Saffron and principal crocetin glycosides. Longhua Chin Med 2022;5:28.