A narrative review on the main chemical constituents and bioactivity of Camellia nitidissima Chi
Introduction
Camellia nitidissima Chi (C. nitidissima) is a precious plant species with high ornamental value because of its canary yellow flowers (1,2). Wild C. nitidissima is predominately distributed in the mountains of Southwest China (particularly in Guangxi Zhuang autonomous region) and North Vietnam (3,4). C. nitidissima has been introduced and cultivated in Japan, Australia, North America, and several provinces in China (5). Guangxi is the main planting area of C. nitidissima in China and the acreage reaches over 2,000 hectares. The annual productions of C. nitidissima fresh leaves and flowers in Guangxi are ~15,000 and ~225 tons, respectively. The economic value of the C. nitidissima industry exceeds 2 billion RMB per year, making it one of the pillar industries in Guangxi (source from the forestry bureau of Fangchenggang City, Guangxi, China).
C. nitidissima was first discovered by the Chinese botanist Jinglie Zuo in Fangchenggang City (Guangxi, China) in 1933, initially named as Theopsis chrysantha Hu by the Chinese botanist Jingwen Qi in 1948 (5), and later revised as Camellia nitidissima Chi in Flora Reipublicae Popularis Sinicae (6). The plant belongs to Theaceae Camellia section Chrysantha Chang, genetically close to Camellia sinensis, Camellia semiserrata, and Camellia oleifera.
Although botanically classified in the last century, C. nitidissima has a long history of use in traditional medicine. It is recorded in Ben Cao Gang Mu, a sixteenth-century Chinese encyclopedia of medical matter and natural history. It exhibits activities in detoxifying, promoting diuresis, and reducing puffiness. It also helps in the treatment of dysentery and pharyngitis. Besides its medical use, C. nitidissima is utilized in daily life for beverages. Freeze-dried C. nitidissima flowers are the most common product of C. nitidissima in the commercial market (Figure 1A). C. nitidissima leaves (Figure 1B) are less popular, which are mainly consumed by ethnic minorities in Guangxi to prepare decoctions for the nourishment. In 2010, C. nitidissima was approved as a new resource food by the Ministry of Health of China, providing a bright future for its applications in medicinal food and dietary supplements.
Phytochemical studies have shown that C. nitidissima contains a variety of active ingredients, such as phenolic compounds, saponins, polysaccharides, volatiles, mineral elements, and amino acids (7). Biological studies have demonstrated that C. nitidissima exhibits antioxidant and anticancer activities in vitro and in vivo (7). In addition, C. nitidissima exhibits lipid-lowering and immunomodulatory activities in animal models (8). In the past decade, several novel compounds in C. nitidissima have been identified and proved to be bioactive (7). In the review, research progresses on the constituents and health-beneficial properties of C. nitidissima in recent years are summarized. It is hoped that the review will cause more readers’ interest in C. nitidissima and inspire them to think about the future prospects of C. nitidissima. It is also hoped that the review will help scientists find out the promising directions for further researches and applications of C. nitidissima.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-9/rc).
Methods
The references in this review are mainly collected from published books and the scientific literature databases including Web of science, PubMed, and China National Knowledge Infrastructure (CNKI), with a timeframe from January 1986 to March 2022, containing English and Chinese references. The search was conducted between January 3, 2022 to March 8, 2022. Search terms included “Camellia nitidissima” and its Chinese characters “金花茶”. The detailed search strategy is listed in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | January 3, 2022–March 8, 2022 |
| Databases and other sources searched | Databases: Web of science, PubMed, and China National Knowledge Infrastructure (CNKI) Published books |
| Search terms used | Search terms: Camellia nitidissima (for English databases, including Web of science and PubMed), 金花茶 (for the Chinese database CNKI) |
| Timeframe | From January 1986 to March 2022 |
| Inclusion and exclusion criteria | Inclusion and exclusion criteria: (I) articles in English and Chinese languages; (II) article types were research articles and reviews |
| Selection process | Hanyu Zheng and Ying Gao conducted the selection together and consensus was obtained after a discussion among all authors |
Main chemical constituents of C. nitidissima
Nowadays, it is generally accepted that herbs play an important role in the prevention and treatment of diseases by virtue of its functional ingredients. There are many studies describing the chemical constituents in C. nitidissima flowers and leaves (1,2,8). Comparatively, less is known about the chemical constituents in other parts of C. nitidissima. Though different in contents, the classes of compounds found in flowers and leaves are similar, including phenolic compounds, saponins, polysaccharides, and other substances.
Phenolic compounds
Phenolic compounds are a diverse group of bioactive secondary metabolites characterized by their structures having at least one phenol unit. Flavonoids, phenolic acids, and lignans are important phenolic compounds in C. nitidissima.
Flavonoids
Flavonoids are low-molecular-weight polyphenolic substances characterized by the flavan nucleus. Many flavonoids are with physiological functions such as antioxidant, antiviral, anti-inflammatory, hypotensive, and lipid-lowering activity (9). Flavonoids are classified into 12 major subclasses, six of which, namely flavonols, flavones, flavan-3-ols, anthocyanidins, flavanones, and isoflavones, are widely distributed in the diet.
The flavonoid content of C. nitidissima is not the highest among members in Theaceae Camellia section Chrysantha Chang (10,11). In C. nitidissima, more flavonoids are accumulated in flowers rather than leaves. A research showed that the flavonoid content of C. nitidissima flowers reached 8.5%, which was 37 times to that of leaves. The flavonoid content of C. nitidissima flowers varies in different stages, decreased in the order of semi-open stage > fish-mouth stage ≈ blooming stage > withering stage ≈ budding stage (12). The flavonoid content of C. nitidissima leaves decreases as the leaves grow. Compared with young leaves, about 69% and 77% of flavonoids were lost in one-year-old leaves and two-year-old leaves, respectively (13). C. nitidissima contains more water-soluble flavonoids than alcohol-soluble flavonoids. Tang (11) found that the flavonoid content of C. nitidissima water extract was much higher than that of C. nitidissima alcohol extract. At present, quite a number of flavonoids in C. nitidissima have been identified.
Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one and flavonols are a class of flavonoids that have the 3-hydroxyflavone backbone. Kaempferol, quercetin, apigenin, and their glycosides are main flavones and flavonols in C. nitidissima. Aromadendrin, dihydroquercetin, dihydrokaempferol, and isorhamnetin glucosides are also detected (2,14,15). Glucose and rhamnose are the two most common sugars to form glycosides in C. nitidissima. Peng et al. (16) used repeated silica gel column chromatography, Sephadex LH-20 column chromatography, ODS column chromatography, repeated recrystallization and other strategies to separate and purify the chemical components of C. nitidissima. Seven of the thirteen obtained compounds belonged to flavones and flavonols, including quercetin, quercetin-7-O-β-D-glucopyranoside, quercetin-3-O-β-D-glucopyranoside, rutin, vitexin, kaempferol, and kaempferol-3-O-β-D-glucopyranoside. In addition to usual monoglycosides, several diglycosides and triglycosides are identified. Some glycosides are modified with the acetyl moiety and/or coumaroyl moiety. For example, Yang et al. (17,18) isolated two acetyl flavonol glycosides from C. nitidissima flowers, namely kaempferol 3-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1/3)-2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1/6)]-β-D-glucopyranoside and kaempferol 3-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1/3)-4-O-acetyl-α-L-rhamnopyranosyl-(1/6)]-β-glucopyranoside, which showed remarkable inhibitory effects on the advanced glycation end-products (AGEs) formation. To be mentioned, a glycoside dimer called kaempferol-3-O-glycosyl-4'-kaempferol-3-O-glycoside was identified in C. nitidissima flowers, which was rare in other plants.
Flavan-3-ols are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Epicatechin and catechin, two flavan-3-ols distributed in many plants, are found in C. nitidissima. Gallocatechin gallate, epigallocatechin (EGC), epicatechin gallate (ECG), catechin gallate, epigallocatechin gallate, and gallocatechin, which are unusual in plants out of the Theaceae family, are detected in C. nitidissima flowers (19). Among these catechins, the abundance of epicatechin is the highest in flowers, followed by EGC and ECG. The contents of catechins in different parts of flowers are not the same. Stamens and petals contain less catechins than sepals. In leaves, catechin and epicatechin are also detected (20). However, the contents of catechins and epicatechins in leaves are much lower than that in flowers. Old leaves accumulate more catechins than young leaves (21). Besides catechin monomers, procyanidins, which refer to the polymers of catechins, exist in C. nitidissima as well. So far, procyanidin dimers, trimers, tetramers, and pentamers have been identified in C. nitidissima flowers (22). Yang (23) identified a unique procyanidin tetramer in C. nitidissima flowers, which was catechin-4→8-catechin-4→8-catechin-3→7-catechin, and named it nitidissimol A.
Anthocyanins are water-soluble vacuolar pigments which are responsible for the vivid colors in plant tissues. Compared with C. nitidissima flowers, more anthocyanins are in leaves. Young leaves have more anthocyanins than old leaves. Li et al. (21) identified two anthocyanins in C. nitidissima, which were pelargonium-3-O-glucoside and cyanidin-3-O-glucoside. The former one existed in both flowers and leaves of C. nitidissima. The latter one was only detected in leaves and was considered to contribute to the purple color of new leaves.
Phenolic acids
Phenolic acids are phenols that contain a carboxylic acid. Gallic acid, chlorogenic acid, salicylic acid, and protocatechuic acid, which are common phenolic acids in plants, are found in the flowers of C. nitidissima (23). Ellagic acid is a phenolic acid with outstanding antioxidant and anti-proliferative properties. Multiple ellagic acid derivatives are identified in the leaves of C. nitidissima. Yu (24) demonstrated the presence of ellagic acid and four ellagic acid derivatives, including 3'-methy-4'-glucoside-ellagic acid, okicamelliaside, 3'-methyellagic acid, and 3,4-O,O-methylidyne-ellagic acid, in the leaves of C. nitidissima. Mo et al. (25) identified five ellagic acid derivatives in the leaves of C. nitidissima, which were 3,4-methylenedioxy-3'-O-methyl-4'-O-(6'-O-acetyl-glucoside) ellagic acid, okicamelliaside, 3,4-O,O-methylidyne-ellagic acid, ellagic acid-4-O-β-D-glucopyranoside, and 3,4-methylenedioxy-3'-O-methyl-4'-O-glucoside ellagic acid. Among these compounds, okicamelliaside is the relatively abundant one, whose content ranges from 0.51% to 1.33% (26). Notably, little study on the ellagic acid derivatives in C. nitidissima flowers was found.
Lignans
Lignans are known to be minor constituents of many plants, often recognized as phytoestrogens. Lignans are derived from phenylanaline, consisting of two phenol units linked by four carbons. Lignans can polymerize to lignin to building the plant cell wall. Lignans often occur in the glycosidic form. They can be metabolized by intestinal bacteria to form mammalian lignans, which have cytostatic activity (27). Zhang et al. (28) isolated and purified lignans from C. nitidissima flowers using silica gel, Sephadex LH-20 gel, C18 reversed silica gel, and semi-preparative high performance liquid chromatography (HPLC). Eight lignans were identified, which were eudesmin, (+)-diasyringaresinol, (+)-isoeucommin A, pinoresinol 4-O-glucoside, 7S, 8R, 8'R-(-)-lariciresinol-4'-O-D-glucopyranoside, (+)-isolariciresinol 9-O-β-D-glucopyranoside, (+)-isolariciresinol 9'-O-β-D-glucopyranoside, and 3', 4-O-dimethylcedrusin. All of them have been reported to possess bioactivity.
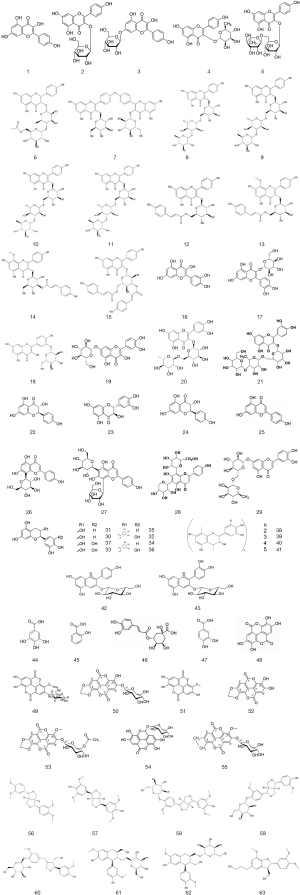
Based on current studies, a table (Table 2) concludes phenolic compounds observed in C. nitidissima are prepared. Their molecular structures are presented in Figure 2.
Table 2
| Classes/compound No. | Compound names | References |
|---|---|---|
| Flavonoids | ||
| Flavonols | ||
| 1 | Kaempferol | (16) |
| 2 | Kaempferol-3-O-β-D-glucoside | (23) |
| 3 | Kaempferol-7-O-β-D-glucoside | (23) |
| 4 | Kaempferol-3-O-rhamnoside | (23) |
| 5 | Kaempferol-3-O-β-D-rutinoside | (23) |
| 6 | Multiflorin C | (23) |
| 7 | Kaempferol 3-O-glucosyl-4'-kaempferol 3-O-glycoside | (23) |
| 8 | Kaempferol 3-O-[α-L-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside | (23) |
| 9 | Kaempferol 3-O-[4-O-acetyl-α-L-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside | (23) |
| 10 | Kaempferol 3-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→3)-4-O-acetyl-α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside | (23) |
| 11 | Kaempferol 3-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside | (23) |
| 12 | Kaempferol 3-O-(6"-O-trans-p-coumaroyl)-β-D-glucopyranoside | (23) |
| 13 | Kaempferol 8-methoxy-3-O-(6"-O-trans-p-coumaroyl)-β-D-glucopyranoside | (23) |
| 14 | Kaempferol 8-methoxy-3-O-(3"-O-trans-p-coumaroyl)-β-D-glucopyranoside | (23) |
| 15 | Kaempferol 3-O-(3",6"-O-trans-p-coumaroyl)-β-D-glucopyranoside | (23) |
| 16 | Quercetin | (23) |
| 17 | Quercetin-3-O-β-D-glucoside | (23) |
| 18 | Quercetin-3'-O-β-D-glucoside | (23) |
| 19 | Quercetin-7-O-β-D-glucoside | (23) |
| 20 | Rutin | (23) |
| 21 | Quercetin-3-O-[α-L-rhamnosyl-(1→2)-β-D-glucosyl]-5-O-β-D-glucoside | (9) |
| 22 | Dihydrokaempferol | (2) |
| 23 | Dihydroquercetin | (2) |
| 24 | Aromadendrin | (14) |
| Flavones | ||
| 25 | Apigenin | (24) |
| 26 | Vitexin | (7) |
| 27 | Apigenin 6,8-di-C-β-glucopyranoside | (24) |
| 28 | Apigenin 6-C-pentoside-8-C-hexoside | (9) |
| 29 | Luteolin-7-O-rutinoside | (24) |
| Flavan-3-ols | ||
| 30 | Epicatechin | (19) |
| 31 | Catechin | (19) |
| 32 | Gallocatechin gallate | (19) |
| 33 | Epigallocatechin | (19) |
| 34 | Epicatechin gallate | (19) |
| 35 | Catechin gallate | (19) |
| 36 | Epigallocatechin gallate | (19) |
| 37 | Gallocatechin | (19) |
| 38 | Procyanidin dimer | (22) |
| 39 | Procyanidin trimer | (22) |
| 40 | Procyanidin tetramer | (22) |
| 41 | Procyanidin pentamer | (22) |
| Anthocyanins | ||
| 42 | Pelargonium-3-O-glucoside | (21) |
| 43 | Cyanidin-3-O-glucoside | (21) |
| Phenolic acids | ||
| 44 | Gallic acid | (20) |
| 45 | Salicylic acid | (23) |
| 46 | Chlorogenic acid | (23) |
| 47 | Protocatechuic acid | (23) |
| 48 | Ellagic acid | (24) |
| 49 | 3'-Methy-4'-glucoside-ellagic acid | (24) |
| 50 | Okicamelliaside | (24) |
| 51 | 3'-Methyellagic acid | (24) |
| 52 | 3,4-O,O-Methylidyne-ellagic acid | (24) |
| 53 | 3,4-Methylenedioxy-3'-O-methyl-4'-O-(6'-O-acetyl-glucoside) ellagic acid | (25) |
| 54 | Ellagic acid-4-O-β-D-glucopyranoside | (25) |
| 55 | 3,4-Methylenedioxy-3'-O-methyl-4'-O-glucoside ellagic acid | (25) |
| Lignans | ||
| 56 | Eudesmin | (28) |
| 57 | (+)-Diasyringaresinol | (28) |
| 58 | (+)-Isoeucommin A | (28) |
| 59 | Pinoresinol 4-O-glucoside | (28) |
| 60 | 7S, 8R, 8'R-(-)-lariciresinol-4'-O-D-glucopyranoside | (28) |
| 61 | (+)-Isolariciresinol 9-O-β-D-glucopyranoside | (28) |
| 62 | (+)-Isolariciresinol 9'-O-β-D-glucopyranoside | (28) |
| 63 | 3', 4-O-Dimethylcedrusin | (28) |
Saponins
Saponins are plant-derived organic chemicals that have a foamy quality when agitated in water. Booming evidences indicate that various saponins have biological activity, such as anti-cancer, lipid-lowering, and anti-bacteria (29). Saponins are characterized by their structure containing a steroid or triterpene aglycone and one or more sugar moieties. Steroidal saponins almost exclusively occur in monocotyle-donous angiosperms, while triterpenoid saponins more frequently occur in dicotyledonous angiosperms (30).
Saponins are observed in different organs of C. nitidissima and the saponin contents were flowers > fruit shells > leaves > buds (31). Saponins in C. nitidissima mainly belong to ursane-type tetracyclic triterpenoids, lupane-type pentacyclic triterpenes, and oleanolane-type pentacyclic triterpenes. Su et al. (32) isolated three ginsenosides from C. nitidissima leaves, which were ginsenoside Rg1, ginsenoside F1, and ginsenoside F5. Wei et al. (33) demonstrated the presence of ilexside II in the water extract of C. nitidissima leaves. Mo (34) identified a new dammarane-type saponin from C. nitidissima, i.e. (3β,6α,12β)-3,6,12-trihydroxydammar-24-en-20-yl-2-O-β-D-glucopyranosyl-(2→1)-O-β-D-glucopyranosyl-(2→1)-O-α-L-rhamnopyranoside, and proved its anti-tumor activity. Yang (23) identified an oleanolane-type triterpene from C. nitidissima flowers, i.e., 3-O-β-D-galactopyranosyl-(1→2)-β-D-glucuronopyranosyl-21β,22α-di-O-angeloyl barringtogenol C. Qi (14) identified four saponins from C. nitidissima leaves, i.e., 3β-acetoxy-20-lupanol, 3β,6α,13β-trihydroxyolean-7-one, 22α-angeloyl-A1-barrigenol, and rubiprasin.
Polysaccharides
Polysaccharides are a group of biomolecules that are essential to all living organisms and are structurally composed of aldoses or ketoses linked by glycosidic bonds (35). They are widely distributed in plants, animals, algae, and microorganisms. Polysaccharides have a variety of biological activities, such as antioxidant, anti-hyperglycemia, anti-hyperlipidemia, anti-inflammation, anti-cancer, and immune enhancement (36).
Niu et al. (37) measured the polysaccharide content in the flowers, leaves, buds, and fruit shells of C. nitidissima, which were 32.88, 29.48, 35.89, and 30.02 g/kg, respectively. Tian (38) analyzed the sugar compositions of C. nitidissima polysaccharides, indicating that the C. nitidissima polysaccharides were composed of glucose, galactose, arabinose, mannose, rhamnose, and xylose. The former four monosaccharides were main components, accounting for 31%, 27%, 21%, and 13%, respectively. Some C. nitidissima polysaccharides not only contain monosaccharides, but also combine with galacturonic acid (39). Gong et al. (40) obtained three C. nitidissima polysaccharides, i.e., TPS1, TPS2, and TPS3, using water extraction, alcohol precipitation, and DEAE cellulose anion exchange chromatography. TPS1 is composed of glucose, galactose, and arabinose. TPS2 and TPS3 are composed of rhamnose, galacturonic acid, galactose, and arabinose. TPS3 contained more galacturonic acid than TPS2. Among them, the antioxidant activity of TPS3 was the best. It implies that polysaccharides with higher content of galacturonic acid tend to possess higher antioxidant capacity. One of the mechanisms is that galacturonic acid has electron-withdrawing groups, such as carboxyl and hydroxyl groups, which provide more hydrogen ions to neutralize free radicals (41).
Tian et al. (42) isolated six polysaccharides from C. nitidissima, three of which belonged to neutral polysaccharides and three of which belonged to pectins. Structural analysis suggested that the three pectins were probably composed of a hairy region which had a backbone of alternating galacturonic acid and α-L-rhamnosyl residues and a smooth region which had a backbone of galacturonic acid residues (42). Lin et al. (43) analyzed the structure of a C. nitidissima polysaccharide, and the results suggested that the polysaccharide was composed of a smooth region with highly methyl esterified galacturonic acid residues and three hairy regions with different chemical structures. Due to these structural characteristics, it is no wonder that several C. nitidissima polysaccharides are digestion-resistant. Gong et al. (44) investigated the digestibility of three polysaccharides and found none of them were digestible. However, all of them showed prebiotic activity. They promoted the proliferations of Lactobacillus and Bifidobacterium, and increased the production of short-chain fatty acids.
Others
C. nitidissima contains multiple mineral elements, such as Ca, Mg, Na, K, P, Cu, Fe, Zn, B, Mn, Ni, and Mo. Some trace elements, such as Se, Sr, Cr, Ge, Co, Ga, and V, are also detected.
C. nitidissima are abundant in free amino acids. Zhao et al. (45) found that there were 16 types of amino acids in C. nitidissima leaves, including 7 essential amino acids. The content of free amino acids was 6% in old leaves and 5.37% in young leaves. Essential amino acids accounted for about 42% of total free amino acids in C. nitidissima leaves. The content and composition of free amino acids in C. nitidissima flowers are not quite the same as that in C. nitidissima leaves. Huang et al. (46) revealed that 17 types of amino acids were observed in C. nitidissima flowers. The content of free amino acids in C. nitidissima flowers ranged from was 4.32% to 5.46%, reaching the top at the fish-mouth stage. Essential amino acids accounted for 38% of total free amino acids in C. nitidissima flowers.
Volatiles are components which contribute to the aroma. Some volatiles also act as bioactive compounds, playing roles in anti-bacteria, anti-virus, anti-depression, and so on. Though almost odorless, 45 volatiles were identified in C. nitidissima flowers (47). Elaidic acid, palmitic acid, and stearic acid accounted for over 30% of total volatiles. (E,E)-2,4-heptadienal, (E,E)-2,4-decadienal, and geranyl acetone, each possessed over 1.7% of total volatiles. Huang et al. (48) identified 37 volatiles in C. nitidissima leaves. Benzoic acid-2-hydroxy-methyl ester was the major volatile, accounting for 26.91% of total volatiles. Benzyl alcohol, cis-octahydropentalene, cis-linaloloxide, phenylethyl alcohol, and 2,6-dimethyl-3,7-octadiene-2,6-diol were relatively abundant in C. nitidissima leaves.
Phytosterols are a family of molecules related to cholesterols and serve as structural components of biological membranes of plants. α-spinasterol, α-spinasteryl-β-D-glucopyranoside, β-sitosterol, and stigmasta-7,22-diene-3-O-[α-L-arabinopyranosyl(1→2)]-β-D-galactopyranoside are observed in C. nitidissima (14,23).
Main bioactivity of C. nitidissima
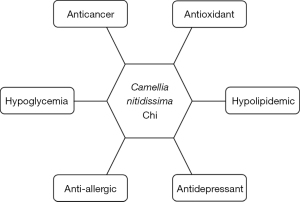
Although C. nitidissima has been traditionally used as an herbal medicine and regarded as health-beneficial, scientific researches on the bioactivity of C. nitidissima have been merely conducted in recent two decades. Evidences indicate that C. nitidissima is potent in antioxidant, anti-cancer, anti-hyperglycemia, and anti-hyperlipidemia (7). Particularly, it works excellent in anti-allergy and anti-depression (7). In next subsections, the main bioactivity (Figure 3) and possible underlying mechanisms are introduced.
Antioxidant activity
At present, there are many studies on the antioxidant activity of C. nitidissima. Wei et al. (49) proved that the ethanol extract of C. nitidissima leaves had hydroxyl radical (·OH) scavenging, superoxide anion radical (O2·−) scavenging, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH·) scavenging activity and reducing power. Qin et al. (50) found that the water extract of C. nitidissima leaves dose-dependently scavenged ·OH and O2·−. At the concentration of 1.25 mg/mL, O2·− was completely scavenged. Yang et al. (18) found that the n-butanol extract of C. nitidissima flowers had a strong inhibitory effect on AGEs. Wen et al. (51) used 95% ethanol to extract the leaves, stamens, buds and petals of C. nitidissima. All the above parts had antioxidant activity, and the buds had the strongest antioxidant activity while the leaves had the weakest.
Phenolic compounds are vital for the antioxidant activity of C. nitidissima. The C. nitidissima flavonoids showed good antioxidant activity with an IC50 of 0.070 mg/mL for the scavenging of DPPH· and an IC50 of 0.679 mg/mL for the scavenging of ·OH (52). Song et al. (15) analyzed the total phenolic content and antioxidant capacity of six types of C. nitidissima leaves by HPLC and liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS), and concluded that the antioxidant capacity was correlated with the total phenolic content. Kaempferol derivatives and quercetin derivatives, well-known for their antioxidant activity, were abundant in C. nitidissima and possibly contributed to the superior antioxidant activity of C. nitidissima. Song et al. (15) found that the dichloromethane and ethyl acetate fractions of C. nitidissima were similarly effective in inhibiting the formation of AGEs in the bovine serum albumin (BSA)-glucose reaction system, while the ethyl acetate fraction was more effective in inhibiting the formation of AGEs in the BSA-methylglyoxal reaction system. Mono- and di-methylglyoxal quercetin adducts were detected in the reaction systems, suggesting that quercetin derivatives inhibited the formation of AGEs by scavenging methylglyoxal. Catechins inhibited the formation of AGEs using the same strategy, i.e., by reacting with methylglyoxal to form adducts (23).
Saponins also play a role in the antioxidant activity of C. nitidissima. Ning et al. (53) used XAD16 macroporous adsorbent resin to isolate and purify C. nitidissima saponins. The crude saponin extract effectively scavenged a variety of free radicals and even worked better than Vitamin C in scavenging ·OH and H2O2. Su et al. proved that ginsenoside F1, which was extracted from C. nitidissima leaves, protected HepG2 cells from H2O2-induced oxidative damage by increasing the superoxide dismutase (SOD) activity (54,55). The results provided a scientific basis for the effectiveness of C. nitidissima on antioxidation in cells.
Polysaccharides from C. nitidissima show antioxidant activity as well. Song et al. (56) isolated three polysaccharides with the β-pyranose configuration from C. nitidissima leaves and demonstrated that the antioxidant activity of neutral polysaccharide was weaker than that of the two acidic polysaccharides, implying the polarity affected the antioxidant activity of polysaccharides. He (41) proved that C. nitidissima polysaccharides with higher content of glucuronic acid exhibited stronger antioxidant activity, which fitted the theory.
Anticancer activity
Multiple researches have proved that C. nitidissima exhibits its anticancer activity not only by preventing the initiation and promotion of cancer, but also the progression of cancer.
C. nitidissima has the potential to be a chemoprevention agent. Daily consumption of diet containing 5% C. nitidissima leaves or 5% C. nitidissima leave extract significantly decreased diethylnitrosamine-induced precancerous lesion of liver in rats (57). Daily intra-gastric administration of C. nitidissima leave extract for 73 weeks effectively reduced the incidence of aflatoxin B1-induced hepatocellular carcinoma and delayed aflatoxin B1-induced hyperplasia in rats (58). The underlying mechanisms included the inhibition of C. nitidissima leave extract on cytochrome P450 enzyme 3A4 (CYP3A4) and glutathione S-transferase π (GST-π), two enzymes mediating the metabolism of aflatoxin B1, as well as the down-regulation of the aflatoxin B1-induced expression of signal transducer and activator of transcription 3 (STAT3), a transcriptional factor well-validated to promote tumorigenesis. Moreover, Sai (59) found that the intra-gastric administration of C. nitidissima flower extract for 16 weeks reduced the incidence of lung tumors by about 13% in an uratan-treated mouse model. The catalase and SOD activity were increased while the malondialdehyde level was decreased in the C. nitidissima flower extract group. The interleukin-2 and tumor necrosis factor α levels were also increased. It suggested that C. nitidissima flower extract exerted the chemopreventive activity via enhancing the antioxidant and immunomodulatory activity of mice.
In addition to preventing cancer, C. nitidissima directly inhibits cancer via affecting the proliferation, apoptosis, cell cycle, and migration of cancer cells.
C. nitidissima reduces the proliferation of various cancer cells in vitro, for example, gastric carcinoma MGC-803 cells (60), esophageal squamous carcinoma Eca-109 cells (61), leukemia U937 cells (62), cervical carcinoma Hela cells (63) and prostate cancer PC-3 cells (64). The effective parts of C. nitidissima include flowers, leaves, and seeds. Yu et al. (65) showed that the alcoholic extracts of C. nitidissima flowers, seeds, and leaves inhibited the proliferation of U937 cells, and the first two extracts also dose-dependently inhibited human colon cancer HCT116 cells.
C. nitidissima extracts promote the apoptosis of cancer cells. Zhao (63) found that C. nitidissima flower extract time-dependently and dose-dependently triggered the apoptosis of Hela cells. Sai (59) demonstrated that the C. nitidissima flower extract upregulated the expression of Bax, a pro-apoptotic protein in the Bcl-2 family, led to the depolarization of the mitochondrial membrane potential, initiated the mitochondrial apoptotic pathway, and eventually caused the intrinsic apoptosis of lung carcinoma A549 cells.
In some cases, C. nitidissima extracts induce cell cycle arrest of cancer cells. Li (60) proved that C. nitidissima flower extract dose-dependently halted MGC-803 cells at the S and G2 cell cycle phases. Shen (66) demonstrated that C. nitidissima ethanol extract blocked the cell cycle of nasopharyngeal carcinoma CNE-2 cells at the G1 phase, induced apoptosis by activating Caspase-3, and down-regulated the expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3, two molecules which were associated with the migration of CNE-2 cells.
At present, most researches on the anticancer property of C. nitidissima are carried out using crude extracts. Only a few studies pinpoint the anticancer components of C. nitidissima. Learning from current studies, saponins may be key anticancer components in C. nitidissima. Mo (34) proved that a dammarane-type saponin from C. nitidissima effectively inhibited the growth of Bel-7402 and SMMC-7721 cells in vitro. Jing et al. (8) verified that 3β,6α,13β-trihydroxyolean-7-one, which was a saponin extracted from C. nitidissima, showed potential cytotoxic activity against SGC7901 cells in vitro. 22α-Angeloyl-A1-barrigenol, another saponin from C. nitidissima, significantly inhibited A549 cells, human gastric carcinoma HGC-27 cells, human breast cancer MDA-MB-435 cells, and human colorectal cancer SW620 cells (8).
Although C. nitidissima has toxicity to cancer cells, it does not hurt normal cells. Li (67) found that C. nitidissima flowers extract had no toxic side effects on human normal liver HL-7702 cells. Sai (59) demonstrated that C. nitidissima extract had no subchronic toxicity in mice. The above results reveal that C. nitidissima is highly selective and effective in inhibiting cancer cells, meanwhile it has low toxicity to normal cells and causes little side effects. It suggests that this plant has a great potential as an anticancer drug candidate.
Hypoglycemic and hypolipidemic activity
Overnutrition is a form of malnutrition in which the intake of nutrients is oversupplied (68). It adversely affects health, causing symptoms like hypoglycemia and hypolipidemia. It may further increase the risks of chronic metabolic diseases, such as diabetes and atherosclerosis.
C. nitidissima leave extracts have excellent hypoglycemic activity. C. nitidissima leave n-butanol and ethyl acetate extracts increased the glucose consumption of insulin-resistant HepG2 cells and decreased the fasting blood glucose and postprandial blood glucose levels in type 2 diabetic mice (69). In another type 2 diabetic mouse model, intra-gastric administration of C. nitidissima leave extract for 28 days increased the insulin level, attenuated pancreatic injury, and promoted the accumulation of hepatic glycogen (70). Feng et al. investigated the effect of C. nitidissima leave extract capsules on lowering blood glucose in diabetic patients. The results supported that C. nitidissima leave extract capsules were effective for the adjuvant therapy of diabetes (71).
C. nitidissima flower extracts display hypolipidemic activity. C. nitidissima flower extract significantly decreased oleic acid-induced lipid accumulation in HepG2 cells by inhibiting the mRNA expression of lipogenesis-related fatty acid synthase, 3-hydroxy-3-methyl glutaryl coenzyme A reductase, and glycerol-3-phosphate acyltransferase genes. It significantly reduced the total triglycerides, total cholesterols, and low-density lipoprotein cholesterols, while increased the high-density lipoprotein cholesterols in serum of hyperlipidemic mice (72). Phenolic compounds may play an important role in it. Zhang (9) observed that C. nitidissima flower flavonoid extract decreased food intake by upregulating the secretion of glucagon-like peptide-1, a hormone negatively regulating the appetite. It inhibited the activity of α-amylase, α-glucosidase, pancreatic lipase, and cholesterol esterase, and decreased the solubility of cholesterol micelles, thus interfering the digestion and absorption of carbohydrates and lipids. In high-fat-diet-induced rats, it reduced lipogenesis, promoted lipolysis and lipid oxidation, attenuated triglycerides and cholesterol accumulation in serum and liver, and alleviated hepatic lipotoxicity. It improved impaired glucose tolerance and restored insulin sensitivity. Additionally, it alleviated high-fat diet-induced dysbiosis.
Anti-allergic activity
Allergy is a number of conditions caused by hypersensitivity of the immune system. Type I hypersensitivity, known as the immediate-type reaction, can be triggered by pollen, foods, drugs, and insect stings. It involves immunoglobulin E (IgE)-mediated release of antibodies against the antigen, degranulation of mast cell, and release of inflammatory factors (e.g., histamine), resulting in symptoms like itch, edema, and pain (73).
C. nitidissima leave water extract and C. nitidissima fruit peel ethyl acetate extract effectively alleviated ovalbumin and Al(OH)3 mixture-induced type I allergy in mice (74). The serum IgE and leukotriene levels were reduced, the number of eosinophils in blood and bronchoalveolar lavage fluid were decreased, and the inflammation in lung was attenuated.
Okicamelliaside, an ellagic acid derivative which exists in Camellia japonica and C. nitidissima leaves, is considered to be the major anti-allergic agent in C. nitidissima. It was 12,000 times more potent than ketotifen fumarate, an antihistamine drug, in inhibiting the degranulation of RBL-2H3 cells (75). It significantly inhibited the vascular hyperpermeability in a passive cutaneous anaphylaxis mouse model. Further study indicated that okicamelliaside inhibited antigen-IgE-FcεRI-induced activation of the Lyn-Syk-LAT-PLCγ-1 pathway, blocked the release of Ca2+, decreased the expression of proinflammatory cytokines (e.g., interleukin-4 and interleukin-13), cytokine-producing signaling factors, and prostaglandin-endoperoxidase 2, resulting in the suppression of allergic inflammation.
Kaempferol 3-O-β-D-glucosyl(1→3) [α-L-rhamnosyl(1→6)]-(2-O-E-p-coumaroyl-β-D-glucoside), a flavonol glucoside obtained from C. nitidissima water extract, is another promising anti-allergic agent in C. nitidissima. It significantly inhibited lipoxygenase activity and leukotriene production in vitro (76).
Antidepressant activity
Depression is a common but serious mood disorder. It causes a persistent feeling of sadness and loss of interest. Nowadays, the stress of life increases. Along with it, is the increasing incidence of depression. Current clinical antidepressant drugs are chemical synthetic drugs, which shows several side effects. Therefore, novel antidepressant drugs with less toxic side effects have come into the limelight.
C. nitidissima contains a variety of natural active ingredients with antidepressant effects, such as quercetin (77), kaempferol (78) and ginsenoside Rg1 (79). C. nitidissima extract significantly decreased corticosterone-induced apoptosis of differentiated PC12 neuronal cells by increasing the expression of brain-derived neurotrophic factor (BDNF) via the protein kinase A-cAMP-response element binding protein signaling pathway (80). It indicated that C. nitidissima extract was capable of protecting neurons. In a chronic unpredictable mild stress rat model, C. nitidissima extract alleviated the decrease of body weight and loss of interest in sucrose. Immunohistochemistry staining and Hematoxylin and Eosin staining confirmed that C. nitidissima extract attenuated the hippocampus injury by increasing the expression of BDNF. Serum corticosterone and adrenocorticotropic hormone, which were increased under depression, was decreased. At the same time, serum SOD and glutathione peroxidase activity were increased while serum malondialdehyde levels were decreased, implying C. nitidissima extract attenuated depression-induced oxidative stress in the body. In mice, C. nitidissima extract also effectively alleviated the depression symptoms. Compared with mice in the model group, the brain and serum serotonin, dopamine, and norepinephrine levels of mice administering C. nitidissima extract were increased. These results suggest that C. nitidissima extract displays its antidepressant activity via multiple targets and it has the promise to be applied in the treatment of depression.
Conclusions
C. nitidissima, though merely being taxonomically classified within a century, has a long history being used as an herb. Chemical analysis reveals that phenolic compounds, saponins, and polysaccharides are important components in C. nitidissima. Some of them are unique in C. nitidissima (e.g., nitidissimol A and some complex flavonol glucosides), some are featuring components in the Camellia genus (e.g., okicamelliaside), and some are commonly distributed in plants (e.g., kaempferol, quercetin, and epicatechin). Biological experiments prove that C. nitidissima exhibits multiple physiological functions, particularly in antioxidant, anti-cancer, anti-hyperglycemia, anti-hyperlipidemia, anti-allergy, and anti-depression. Scientific evidences of the pharmacological value of C. nitidissima and corresponding chemical basis are partially established.
It is noteworthy that most of the current biological studies of C. nitidissima are based on crude extract without a clear description of the chemical profile, which makes it difficult to figure out the predominant bioactive component. Some bioactivity of C. nitidissima is verified in vitro, whether it works in vivo or not still remains unknown. In addition, current understanding of the molecular mechanisms of C. nitidissima is relatively preliminary. In the future, more attentions should be drawn on the bioactivity of individual component in C. nitidissima. The assessments are recommended to be conducted both in vitro and in vivo. Detailed working mechanisms are encouraged to be explored. Researches of pharmacokinetics and pharmacodynamics are also necessary. By ascertaining these properties of individual component, investigations on interactions between bioactive components can be carried out more easily. The above information will help us better understand why C. nitidissima is capable of a specific physiological function and how it exhibits the activity.
It is also aware that some empirical therapeutic activity of C. nitidissima still lacks scientific proof. Little is known about the activity of some special compounds in C. nitidissima. Future researches on these aspects are needed. The results will certainly enhance the knowledges of C. nitidissima and benefit the applications of C. nitidissima in health industry.
Acknowledgments
Funding: This research was supported by the China Agriculture Research System of MOF and MARA (CARS-19) and the Innovation Project for the Chinese Academy of Agricultural Sciences.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-9/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-9/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou XW, Fan ZQ, Chen Y, et al. Functional analyses of a flavonol synthase-like gene from Camellia nitidissima reveal its roles in flavonoid metabolism during floral pigmentation. J Biosci 2013;38:593-604. [Crossref] [PubMed]
- Wang W, Liu H, Wang Z, et al. Phytochemicals from Camellia nitidissima Chi inhibited the formation of advanced glycation end-products by scavenging methylglyoxal. Food Chem 2016;205:204-11. [Crossref] [PubMed]
- Chang B, Huang G. The classification and geographic distribution of Camellia nitidissima. Journal of Wuhan Botanical Research 1986;4:31-42.
- Zhang X, Feng J, Su S, et al. Hepatoprotective effects of Camellia nitidissima aqueous ethanol extract against CCl4-induced acute liver injury in SD rats related to Nrf2 and NF-κB signalling. Pharm Biol 2020;58:239-46. [Crossref] [PubMed]
- Liang SY, Lu MZ, Huang LD. The cultivation and utilization of Camellia nitidissima. Beijing: China Forestry Press, 2005.
- Ye CX. Notes on the change of the scientific name of Camellia nitidissima. Guangxi Plants 1997;4:22-6.
- Dong Y, He, Xiao Y, et al. Camellia nitidissima C.W. Chi: a review of botany, chemistry, and pharmacology. Phytochemistry Reviews Proceedings of the Phytochemical Society of Europe 2018;17:327-49.
- Jing Q, Ruo FS, Jian MY, et al. Chemical constituents from leaves of Camellia nitidissima and their potential cytotoxicity on SGC7901 cells. Chinese Herbal Medicines 2016;8:80-4. [Crossref]
- Zhang HL. Anti-food-induced obesity effect and mechanism of Camellia nitidissima flavonoids. Guangdong Ocean University, 2020.
- Huang YL, Wen YX, Liu JL, et al. Determination of total flavonoids in five kinds of Camellia nitidissima. China Science and Technology of Traditional Chinese Medicine 2009;16:38-9.
- Tang Q, Luo YY, Huang LD, et al. Determination of Chemical Constituents in Section Chrysantha Chang. Lishizhen Medicine and Materia Medica Research 2009;20:769-71.
- Huang YZ, Chen JY, Zhang WJ, et al. Comparison of quality and yield of Camellia nitidissima in different flowering stages. Fujian Agricultural Journal 2021;36:899-908.
- Huang XX, Zou R, Hu XH, et al. Comparison of total flavonoids content in 14 species of Camellia nitidissima sect. Chrysantha. Guihaia 2011;31:281-4.
- Qi J. Isolation, identification and activity evaluation of chemical constituents of Camellia nitidissima. Nanjing University of Science and Technology, 2016.
- Song L, Wang X, Zheng X, et al. Polyphenolic antioxidant profiles of Camellia nitidissima. Food Chem 2011;129:351-7. [Crossref] [PubMed]
- Peng X, Yu DY, Feng BM, et al. Study on the chemical constituents of Camellia nitidissima. Guihaia 2011;31:550-3.
- Yang R, Guan Y, Wang W, et al. Antioxidant capacity of phenolics in Camellia nitidissima Chi flowers and their identification by HPLC Triple TOF MS/MS. PLoS One 2018;13:e0195508. [Crossref] [PubMed]
- Yang R, Wang WX, Chen HJ, et al. The inhibition of advanced glycation end-products by five fractions and three main flavonoids from Camellia nitidissima Chi flowers. J Food Drug Anal 2018;26:252-9. [Crossref] [PubMed]
- Jiang LN, Li JY, Fan ZQ, et al. Analysis of polyphenol components in flowers of Camellia nitidissima plants. Forestry Science Research 2020;33:117-26.
- Yan DM, Li RJ. Determination of 5 kinds of phenolic substances in Camellia nitidissima by high performance liquid chromatography. Journal of Henan University of Technology 2010;31:59-62. (Natural Science Edition).
- Li XL, Wang JT, Sun ZY, et al. UPLC-QTOF-MS analysis of Camellia nitidissima flowers and leaves. Scientific Research in Forestry 2018;31:83-8.
- Zhang HL, Yu QT, Wu QX, et al. On-line screening of flavonoids from Camellia nitidissima and in vivo antioxidant activity based on iron ion interaction combined with high performance liquid chromatography. Research and Development of Natural Products 2020;32:719-26.
- Yang R. The chemical constituents of Camellia nitidissima and its biological activities based on quorum sensing inhibition. Nanjing University of Science and Technology, 2019.
- Yu J. Analysis of polyphenolic compounds in Camellia nitidissima leaves. Guangxi University of Traditional Chinese Medicine, 2017.
- Mo JG, Chen QH, Huang Y, et al. A new ellagic acid compound in Camellia nitidissima. Chinese Herbal Medicine 2018;49:75-9.
- Cheng CJ, Cong LF, Li ZQ, et al. Screening, preparation and investigation of antitumor activity of Okicamelliaside in Camellia nitidissima. Traditional Chinese Medicine in Tianjin 2020;37:1425-30.
- Wcislo G, Szarlej-Wcislo K. Colorectal Cancer Prevention by Wheat Consumption: A Three-Valued Logic-True, False, or Otherwise? Elsevier Inc., 2014.
- Zhang PP, Wang ZN, Yang R, et al. Analysis of chemical constituents of lignans in Camellia nitidissima. Acta Tropical Biology 2020;11:296-300, 323.
- AAT Intégral. Saponins: properties, applications and processing; 2010.
- Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol 2004;94:219-43. [Crossref] [PubMed]
- Niu GJ, Xing JH, Zhu S, et al. Determination of active components and antioxidant activity of Camellia nitidissima. Journal of Forestry and Environment 2015;35:165-8.
- Su L, Mo JG, Wei YL, et al. Study on saponins components of Camellia nitidissima. Chinese Herbal Medicine 2012;43:3.
- Wei JB, Nong CL, Su ZH, et al. A preliminary study on in vitro antitumor activity and material basis of Camellia nitidissima. Chinese Journal of Experimental Formulas 2014;20:169-74.
- Mo JG. Camellia saponin A and its preparation method and antitumor use. Guangxi Zhuang Autonomous Region, Guangxi Zhuang Autonomous Region Analysis and Testing Research Center, 2017.
- Ullah S, Khalil AA, Shaukat F, et al. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019;8:304. [Crossref] [PubMed]
- Yu Y, Shen M, Song Q, et al. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr Polym 2018;183:91-101. [Crossref] [PubMed]
- Niu GJ, Zhu S, Chen QY, et al. Determination and in vitro antioxidant activity of polysaccharides from different parts of Camellia nitidissima. Chinese Journal of Experimental Formulas 2014;20:168-72.
- Tian XC. Isolation, purification and chemical structure study of polysaccharides from Camellia nitidissima. Guangdong Ocean University; 2011.
- Wang DF, Li J, Wang CH. Study on the component and immune activity of polysaccharides from tea. J Tea Sci 2000;20:45-50.
- Gong W, Tang J, Wei YY, et al. Isolation, purification, structural characterization and in vitro antioxidant activity of polysaccharides from Camellia nitidissima. Food and Machinery 2021;37:184-90.
- He Z. Isolation, Purification, Antioxidant and Immune Activity of Ashwagandha Polysaccharide. Beijing: Chinese Academy of Forestry Sciences, 2014.
- Tian XC, Qin XM, Lin HJ, et al. Study on the physicochemical properties of polysaccharides from Camellia nitidissima. Chinese Journal of Foodstuffs 2011;11:47-52.
- Lin HJ, Tian XC, Qin XM, et al. Analysis of chemical structure characteristics of single polysaccharide components in Camellia nitidissima. Food Science 2013;34:6.
- Gong W, Tang J, Wei YY, et al. Study on in vitro digestion and fermentation characteristics of polysaccharides from Camellia nitidissima. Food Industry Science and Technology 2021;42:9.
- Zhao HJ, Luo ZR, Ding YL, et al. Analysis of nutrients in old and young leaves of Camellia nitidissima. Journal of Inner Mongolia Agricultural University (Natural Science Edition) 2016;37:52-6.
- Huang YZ, Chen JY, Zhang WJ, et al. Comparison of quality and yield of Camellia nitidissima in different flowering stages. Fujian Agricultural Journal 2021;36:10.
- Wei Q, Zhang LY. Comparative analysis of two kinds of Camellia nitidissima aroma components. Modern Food Science and Technology 2013;29:668-72.
- Huang YL, Chen YY, Wen YX, et al. GC-MS analysis of volatile components in Camellia nitidissima. Food Science and Technology 2009;34:257-60.
- Wei X, Huang XX, Jiang YS, et al. Comparison of antioxidant activities of plant extracts from three Camellia nitidissima groups. China Journal of Traditional Chinese Medicine 2011;36:639-41.
- Qin XM, Lin HJ, Ning EC, et al. Antioxidant activity of water extracts from Camellia nitidissima. Food Science and Technology 2008;189-91.
- Wen J, Liang W, Wang XC, et al. Study on the chemical constituents and anti-inflammatory and antioxidant activities of Camellia nitidissima. Chinese Journal of Medicinal Chemistry 2020;30:487-92.
- Xu JH, Liu MM, Qi DJ, et al. Optimization of aqueous two-phase extraction of total flavonoids from Camellia nitidissima and analysis of antioxidant activity. Food Industry Science and Technology 2022;43:155-61.
- Ning EC, Xin M, Wei L, et al. Antioxidative activity of saponins from Camellia nitidissima. Food Science and Technology 2009;34:197-9.
- Su L, Mo JG, Wei YL, et al. Chemical constituents of saponins from leaves of Camellia nitidissima. Chinese Traditional and Herbal Drugs 2012;43:877-9.
- Wen L, Guo XB, Liu RH, et al. Phenolic contents and cellular antioxidant activity of Chinese hawthorn “Crataegus pinnatifida”. Food Chem 2015;186:54-62. [Crossref] [PubMed]
- Song LL, Wen G, Huo SH, et al. Isolation, purification, structural characteristics and antioxidant activity of polysaccharides from turmeric. Food and Fermentation Industry 2020;46:73-9.
- Tang XL, Fu JY, Duan XX, et al. Preliminary study on the inhibitory effect of Camellia nitidissima and Ginkgo biloba on 2-ethylnitrosamine-induced precancerous lesions in rats. Journal of Practical Cancer 2007;22:4.
- Ou C, Cao J, Yang C, et al. The chemopreventive effect and mechanism of Camellia nitidissima in inhibiting aflatoxin B1-induced liver cancer in rats. Proceedings of the National Academic Conference on Tumor Epidemiology and Tumor Etiology Collection 2011:131-3.
- Sai X. Preliminary study on the preventive effect of Camellia nitidissima extract against lung cancer and its mechanism. Dalian University of Technology, 2018.
- Li L. Effects of Camellia nitidissima water extract on the proliferation and cycle of human gastric cancer MGC-803 cells. Nanning: Guangxi Medical University, 2013.
- Dai L, Li JL, Liang XQ, et al. Flowers of Camellia nitidissima cause growth inhibition, cell-cycle dysregulation and apoptosis in a human esophageal squamous cell carcinoma cell line. Mol Med Rep 2016;14:1117-22. [Crossref] [PubMed]
- He GL, Wang CY, Tan H, et al. Study on the inhibitory effect of the extract of Camellia nitidissima on human monocytic leukemia cell line U937 cells. Bright Traditional Chinese Medicine 2014;29:1382-4.
- Zhao YH. Effects of Camellia nitidissima water extract on the proliferation and apoptosis of human cervical cancer Hela cells. Lanzhou: Lanzhou University, 2015.
- Han LC, Shi LY, Yu DY, et al. Experimental study on the inhibitory effect of Camellia nitidissima seeds on hormone-related tumors in vitro. Shi Zhen Guo Yi Guo Yao 2009;20:3146-8.
- Yu DY, Shi ZS, Shi LY, et al. Experimental observation on the proliferation inhibition of U937 and HCT116 cells by extracts of Camellia nitidissima, seeds and leaves. Chinese Patent Medicine 2013;35:2005-7.
- Shen J. Camellia nitidissima inhibits the growth of human poorly differentiated nasopharyngeal carcinoma cells and the expression of VEGF-C\VEGFR-3 in vitro. Guilin Medical College, 2011.
- Li CY. Effects of different concentrations of Camellia nitidissima and camellia on precancerous lesions of rat liver induced by diethylnitrosamine. Guangxi Medical University, 2007.
- Mathur P, Pillai R. Overnutrition: Current scenario & combat strategies. Indian J Med Res 2019;149:695-705. [Crossref] [PubMed]
- Chen LM, Wang YJ, Xiao YM, et al. Screening of the hypoglycemic activity of Zhuang medicine Camellia nitidissima. Modern Chinese Medicine Research and Practice 2017;31:5.
- Xia X, Pan CS, Huang L, et al. Effects of Camellia nitidissima on pancreatic function in diabetic mice. Shi Zhen Chinese Medicine and Chinese Medicine 2013;24:3.
- Feng Q, Wang ZP, Xu K, et al. Clinical observation on adjuvant treatment of type 2 diabetes mellitus with Camellia nitidissima Capsule. Guangxi Traditional Chinese Medicine 2015;38:32-3.
- Zhang Ping. Study on the hypolipidemic effect of the flower extract of Camellia nitidissima. Dalian University of Technology, 2015.
- Abbas M, Moussa M, Akel H. Type I Hypersensitivity Reaction 2022.
- Wang YQ, Peng X, Tang Q, et al. Screening of plant anti-IgE-mediated type I allergic reaction in Camellia nitidissima. Zhongnan Pharmacy 2009;7:4.
- Onodera K, Tsuha K, Yasumoto-Hirose M, et al. Okicamelliaside, an extraordinarily potent anti-degranulation glucoside. Biosci Biotech Bioch 2010:2532-4.
- Shi LY, Yu DY. A kind of Camellia nitidissima flavonoid glycoside and its preparation method and use [patent]. Liaoning Province: CN103951723B, 2015.
- Wang YQ, Wang YH, Zou MS, et al. Research progress on the antidepressant effect and mechanism of quercetin and its glycoside derivatives. Chinese Herbal Medicine 2022;53:1548-57.
- Liang YD, Tan YG, Zhang S, et al. Effect and mechanism of kaempferol on depression-like behavior in aged rats with chronic stress and depression. Chinese Journal of Clinical Pharmacology 2020;36:4028-30.
- Huang Q, Chu SF, Lian XY, et al. Antidepressant effect of ginsenoside Rg1 and its mechanism of action. Acta Neuropharmacologica Sinica 2013:1-11.
- He DY. Study on the antidepressant effect of the medicinal leaf extract of Camellia nitidissima. Dalian University of Technology, 2018.
Cite this article as: Zheng H, Du Q, Yin J, Gao Y. A narrative review on the main chemical constituents and bioactivity of Camellia nitidissima Chi. Longhua Chin Med 2022;5:29.