
Probiotics in common urological conditions: a narrative review
Introduction
The advantages of the use of beneficial bacteria or probiotics for human health are immense and this concept is more than hundred years old. Elie Metchnikoff, a Russian scientist had advocated beneficial effects of consumption of Lactobacillus fermented milk to control the disturbed intestinal microflora, which was published in 1907 (1). Probiotics can be defined as “Live microorganisms, which confer a health benefit on the host, when administered in adequate amounts” (2). A good probiotic agent must be nonpathogenic, non-invasive and non-carcinogenic. It should have the capacity to resist the gastric acidity and should remain intact in alimentary canal during intestinal digestion. It should be able to adhere to intestinal epithelium, aid in immunomodulation and stably colonize for a long-term benefit. Moreover, they must be active against a specific target and display bile salt hydrolase activity (3). The probiotics are extensively used for strengthening immunity and protecting the human body against different ailments including urogenital diseases. This could be possible by regular intake or consumption of probiotics as part of our daily diet (4). Substantial life style changes in modern era had exposed us to maximum food preservatives in form of antimicrobials that affected tremendously our natural commensal flora by impairing our immunity and reducing the ability to fight against different infections. This could be counteracted by in taking certain beneficial bacteria or probiotics (Bifidobacteria, Lactobacilli, etc.) that are proven to enhance the immune status and protect our body against bacterial or viral infections. They are considered as safe because they can reside in the human body without causing any harm. Moreover, they are key microbes responsible for food preservation and milk fermentation and were used since antiquity by mankind (5).
Probiotics are effective in preventing and treating various diseases and health ailments like urogenital infections, reducing antibiotic side effects, allergies, lactose intolerance, gastrointestinal (GI) infections, inflammatory bowel disease, cystic fibrosis, various cancers, in oral health such as periodontal diseases, oral malodors and prevention of dental caries, etc. Gram-positive bacteria belonging to two genera, i.e., Bifidobacterium and Lactobacillus were mostly used. Though, probiotics were frequently used to restore intestinal dysbiosis resulted after a prolonged antibiotic therapy yet, they have proven therapeutic and prophylactic role against various urological ailments, which are most common and discomforting health issues among all age groups. Therefore, the present review is conceptualized. The key objective of this topic is to enlist the common urological conditions and their etiological agents, the mechanism of action of the active biomolecules of some frequently used probiotics for various health ailments, the efficacy of probiotics for urinary tract infection (UTI) and specifically bacterial and fungal vaginosis, clinical trials to support such claims and to discuss about the faulty marketing strategies to sell or prescribe certain probiotic goods/products with negligible tangible benefits. We present the following article in accordance with the Narrative Review reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-62/rc).
What are probiotics and their role in general health of human?
Daily, we ingest a huge number of living organisms, mostly bacteria as they are abundantly present everywhere. Though they are often found in food and water, they can also be added to cheese, yoghurt or fermented milk products and sausages during their processing to increase their health benefits. The most commonly used probiotics belong to gram-positive bacilli group. They are facultative aerobes that often generate non-pathogenic spores. These spores or the probiotic products have increased shelf-life due to their thermostable/temperature resistant, resilient nature to pH variations and survivability within the harsh gut environment. The copious peptidoglycan content present on the spores protects them from extreme conditions such as heat, lysosomal degradation and organic acids, etc. The group of Bifidobacteria, Lactobacilli, Leuconostoc Pediococcus and Enterococci are the commensals, normally non-pathogenic and facultative anaerobes. They are found naturally in human intestine, survive effectively and colonize the gut epithelium. They also inhibit other pathogenic bacteria by producing bacteriocins and help in maintaining a healthy microbiota, which are helpful in regulating cholesterol level and treating diarrhea and inflammatory bowel disease (IBD) (6).
Common urological conditions
The urinary system consists of urethra, bladder, ureters and the kidneys. The urinary tract can have problems like any other organs or systems of our body and these problems are usually denoted as urological problems or conditions. Each one of us would have experienced it once or often in our life regardless of our age, gender or ethnicity. Men and women are equally prone to different types of urological conditions that are often linked to how we pass out urine and these conditions also affect the reproductive systems in men (7). The different type of urological condition are listed below;
Urinary incontinence or not able to control/hold urination
There could be varied reasons to have this condition due to spinal cord injury (SCI), weakened muscles of bladder or sphincter muscles, diabetes, childbirth, certain diseases and even severe constipation. Though it is not harmful, it may cause some embarrassing situations and could be a burden on our daily life. Although both men and women can suffer from stress incontinence, it is more common in women. The valve-like muscles regulating the release of urine are weakened in the urethra and they tussle to stay closed (7).
Overactive bladder
This is a condition, when bladder is unable to retain urine appropriately and there is automatic passage of urine due to contraction of bladder muscles and muscle spasm. This results an intense and sudden urge to urinate, which might be due to neurological disorders, diabetes, UTIs, bladder stones, tumors or simply for aging (8).
UTIs
Commonly seen in female due to their anatomy of short urethra and small gap between anus and urethra. Up to sixty percent of women can experience an episode of UTI at any point of their life and only twelve percent of men may experience it (7).
Kidney and ureteral stones
Kidney and ureteral stones are formed due to calcium oxalate crystal formation in urine that blocks the urinary tract and makes urination painful. While most stones are cleared naturally, the larger stones often require either surgery or specific procedures to break them.
Female specific urologic problems
Short urethra and post-partum dysfunction of pelvic muscles can increase UTI, pelvic floor dysfunction, incontinence, etc. (9).
Male specific urologic problems
Anatomical differences between male and female, subjects them to experience unlike urological complications, i.e., benign prostatic hyperplasia (BPH) (enlarged prostate), prostate cancer (second common cause of death in men), prostatitis (abnormal swelling or inflammation of the prostate), erectile dysfunction (difficulty in getting or maintaining an erection), nocturia, hematuria, male infertility, premature ejaculation, testicular cancer, etc. (10).
Methods
Extensive search on the relevant literature published between 2000 and 2021 were conducted by using computerized search engines such as Google, ScienceDirect, PubMed and https://asm.org/, using different keywords like, “probiotics”, “urological conditions”, “urinary tract infections”, “urogenital tract infections”, “bacterial vaginosis”, “antimicrobial activities of probiotics” and “antibiofilm activities of probiotics” using English as screening language and reviews, reports, case studies and original research articles are included in this review. Clinical trials and other important reports published prior to this period were also included. The literature survey pertaining to the subject is presented in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 15-09-2021 to 14-10-2021 |
| Databases and other sources searched | Google, Science Direct, PubMed, and www.asm.org |
| Search terms used (including MeSH and free text search terms and filters) | “Probiotics”, “Urological conditions”, “Urinary tract infections”, “Urogenital tract infections”, “Bacterial vaginosis”, “Antimicrobial activities of probiotics” and “Antibiofilm activities of probiotics” |
| Timeframe | Published between 1975 and 2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | In vivo, in vitro and clinical study research articles published in English were included and articles not coming under the purview of the title were excluded. For reliability, only publications on peer-reviewed journals were incorporated. A total of 87 articles were referred for this review (55 included and 32 excluded) |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Both S Das and S Ameeruddin conducted searches, S Das selected the articles, S Ameeruddin compiled the data, S Das edited and wrote the review article. Both S Das and S Ameeruddin drawn the artworks |
| Any additional considerations, if applicable | None |
Main text
List of probiotics used against common urological conditions
In order to improve the urological conditions, four Lactobacillus strains were frequently used as potential probiotics: Lactobacillus rhamnosus GG, L. rhamnosus GR-1, L. crispatus CTV05 and L. fermentum RC-14. L. rhamnosus GR-1 is resistant to spermicide, greatly adherent to vaginal and uroepithelial cells and able to constrain growth and adhesion of uropathogens and Candida albicans (11,12). L. fermentum RC-14 was another adherent strain highly potent biosurfactant that produces H2O2 and also constrains attachment of a group of uropathogens, which consist of Gardnerella vaginalis, C. albicans and Escherichia coli (11,12). When administered in vaginal capsules, both of them stayed in vaginal microflora of normal pre-menopausal females for a considerably longer period than L. rhamnosus GG (13). Therefore, L. rhamnosus GR-1 and L. fermentum RC-14 might be better agents for vaginal colonization. The properties of L. crispatus CTV05 is not well-known except its ability to produce H2O2 and inhabit the vagina (14). L. crispatus was strongly attached to vaginal epithelium, blocked adhesion of Staphylococcus saprophyticus, E. coli and Proteus mirabilis and inhibited the proliferation of important uropathogens (15); L. crispatus, a strong adherent of vaginal epithelium, blocked the attachment of C. albicans; along with Staphylococcus aureus and Pseudomonas aeruginosa by repressing their growth (16). Bifidobacteria (Bifidobacterium lactis Bb12, Bifidobacterium longum) were also reported to be excellent probiotic agents. The list of probiotics frequently used for various urological conditions is presented in Table 2 and list of pathogens associated with common urogenital problems are presented in Table 3.
Table 2
| Type of ailment | Name of bacteria | Reference |
|---|---|---|
| Antiurobacterial activity | Lactobacillus rhamonosus GG | (11,12) |
| L. rhamonosus GR-1 | ||
| L. cryspatus FTV05 | (14) | |
| L. fermentum RC-14 | ||
| Bacterial vaginosis (BV), aerobic vaginosis (AV) | L. rhamonosus HN001 | (17) |
| L. acidophilus GLA14 | ||
| Recurrent urinary tract infections in children | L. acidophilus | (18) |
| Urinary tract infections | L. fermentum ME-3 | (19) |
| B. longum 46 | ||
| L.acidophilus La5 | ||
| L. plantarum 299v, L. paracasei 8700:2 | ||
| Bifidobacterium lactis Bb12 | ||
| L. fermentum CRL 1058 | (20) | |
| Urolithiasis caused by Proteus mirabilis | L. plantarum | (21) |
| L. brevis |
Table 3
| Type of ailment | Name of bacteria | Reference |
|---|---|---|
| Urinary tract infections (UTI) | Escherichia coli | (11,12,15) |
| Staphylococcus aureus | ||
| Pseudomonas aeruginosa | ||
| Klebsiella pneumoniae | ||
| Enterococcus faecalis | ||
| Proteus mirabilis | ||
| Candida albicans | ||
| Bacterial vaginosis (BV) | Gardnerella vaginalis | (22,23) |
| Mycoplasma hominis | ||
| Megasphaera sp. | ||
| Atopobium vaginae | ||
| Ureaplasma urealyticum | ||
| Prevotella | ||
| Mobiluncus sp. | ||
| Peptostreptococcus sp. | ||
| Vulvovaginal candidiasis (VVC) | Candida sp., Candida albicans | (24,25) |
| Aerobic vaginosis (AV) | E. coli | (17) |
| S. aureus |
Lactobacillus: an ideal probiotic agent
As Lactobacilli are part of normal microflora, they are considered as safe for human use. Mostly, people take them for longer duration without any harmful effects (12,26-28). But a particular individual may be prone to opportunistic infections by the normal flora. Therefore, few investigations were carried out in this regard. Extensive use of L. rhamnosus GG in Finland could not cause Lactobacillus bacteremia (29). Consumption of dairy products every day, especially yoghurt was related to the chances of liver abscesses, endocarditis and bacteremia, even though it is a rare occurrence (30). As molecular studies failed to reveal that the consumed probiotic strains and infective strains were same (12,27). Therefore, they are considered as safe for short or longtime use based on priority to avoid recurrent urogenital tract infections in normal pre-menopausal or post-menopausal females, to re-establish the vaginal flora after an antibiotic therapy and to prevent future episodes of bacterial vaginosis (BV) and recurrent vaginal candidiasis (RVC). This could also be beneficial in high-risk groups for UTI, such as females with structural anomalies of the urinary tract or patients with neurogenic bladder, renal transplant recipients or preoperative patients for gynecological or urological procedures or patients with SCI (31).
Role of probiotics in prevention of urological condition
The female urogenital system comprises of the organs associated with reproduction and production and discharge of urine. Reproductive system consists of ovaries, fallopian tubes, uterus and vagina, while the kidneys, ureters, bladder and urethra constitute the urinary system. Urogenital infections are the most common and challenging health problems in women due to their short and straight urethra that makes it easier for germs to move into the bladder up to the kidneys causing more complications. Unsexually transmitted urogenital infections comprise of UTI, BV and yeast vaginitis (32).
The urogenital system has a healthy resident microflora encompassing aerobic and anaerobic microbes (33), which gets disturbed after a prolonged antibiotic therapy that often needs restoration. Probiotics are used to restore microbial ecosystem and Lactobacilli are commonly used for this. The use of Lactobacilli to restore the microbial flora of the female urogenital tract started in early 1900s (34). But the intake of probiotics or probiotic products for preventing and treating vaginitis and BV become more popular in the beginning of the nineties. As antibiotics are not always effective and often results different side-effects, recurrent infections, bacterial and yeast resistance, alternate therapies are gaining popularity among patients and their healthcare providers. Moreover, probiotics can be used for a longer duration without any antagonistic effects, which make them a striking alternative remedy for resolving high recurrence problems.
The different species belonging to Lactobacillus genus prevail in the vaginal microbiota (32,35,36) that include L. jensenii, L. crispatus, L. gasseri, and L. iners (36-38) along with L. rhamnosus, L. plantarum, L. brevis, L. casei, L. vaginalis, L. acidophilus, L. salivarius, L. fermentum, L. delbrueckii and L. reuteri (35). Lactobacilli protect the vagina against pathogenic microorganisms by creating a biofilm on the vaginal mucosa. They exclude the pathogens by varied mechanisms, like co-aggregation, competition for nutrients, exclusion of epithelium adhesion, stimulation of immune system and inhibit the pathogens by production of antimicrobial substances (H2O2 and bacteriocins) and secretion of some organic acids (39). A healthy and established vaginal microbiota can prevent urological conditions and maintain wellness (40,41). But this could be lost and dysbiosis could be resulted by use of antibiotics, hormonal alterations or through sexual activity (40). BV is a condition of overgrowth of gram-negative or anaerobic organisms led by G. vaginalis, Mycoplasma hominis, Megasphaera species, Atopobium vaginae, Ureaplasma urealyticum, Prevotella, Mobiluncus sp. and Peptostreptococcus sp. (22,23). The common symptoms include malodorous vaginal discharge and irritation (42), which is treated by antibiotics, like clindamycin, metronidazole, secnidazole and tinidazole, administered either orally or intra-vaginally (43). Conversely, due to recurrences of BV and high morbidity after antibiotic therapy, alternative agents are gaining attention. Fungal/yeast vaginitis has symptoms of white vaginal discharge with typical malodor, non-homogenous caseous appearance with vaginal or introitus itch and irritation (44). Vulvovaginal candidiasis (VVC) is an infection initiated by Candida sp. with inflammation in absence of other contagious agents (24,25). Re-establishment of vaginal microbiota and/or augmentation of resident mucosal immune response can be mediated by probiotic food supplements, intra-vaginal suppositories or topical gels (45-47). The effects of L. acidophilus GLA-14 and L. rhamnosus HN001 on pathogens causing BV (G. vaginalis and A. vaginae) and aerobic vaginosis (AV) (S. aureus and E. coli) were assessed. In vitro studies revealed that probiotics inhibit BV and AV causing pathogens and L. acidophilus GLA-14 exerted maximum antagonistic activity against anaerobic bacteria (17).
Infectious urolithiasis or urinary stone formation resulted due to crystallization of mineral salts present in urine. It is mainly induced by urease activity of Proteus mirabilis, the common causative agent of infectious urolithiasis. The effect of L. plantarum and L. brevis strains on P. mirabilis induced salt crystallization was assessed in vitro and it was found that some Lactobacillus strains populated the uroepithelium and executed bactericidal properties (21). The presence of some Lactobacilli enhanced growth of uropathogens and also increased crystallization resulting larger size of crystals. L. plantarum and L. brevis strains increased UTI and augmented the development of urinary stones (21). Therefore, it was suggested that without knowing precisely the complexity of microbial interactions, it is unsafe to introduce any bacteria into the human body in order to prevent or treat diseases.
Chronic UTI enhances the risk of bladder cancer by increasing bladder inflammation and carcinogen production (48). These inflammatory agents produced in the bladder has the potentiality to incur cancer at local and remote places, although the mechanisms are still unclear. Therefore, novel methods are needed to restrain inflammatory actions, as existing neurogenic bladder treatment is dependent on antibiotics and it is impossible to combat the multidrug resistant (MDR) strains present in dense biofilms. Earlier, it was reported that probiotic bacteria have the potency to restrict infections and inflammatory practices underlying in the urogenital tract (12,49,50). Oral ingestion of probiotic strains, i.e., L. rhamnosus GR-1 and L. reuteri (previously fermentum) RC-14 reduced the number of pathogens in the urogenital system (49), and L. acidophilus strain indicated at par efficiency as prophylactic antibiotics in avoiding recurrent UTI in children (18). Direct introduction of Lactobacilli into neurogenic bladder of patients could not colonize the bladder (51). A nonpathogenic E. coli strain when embedded into the bladder of SCI patients, a reduction in the ability of infectious pathogens to colonize the bladder was observed (52). Lactobacilli can be supplemented with antibiotics to treat acute infections (50), and oral supplementation of Lactobacilli can facilitate analgesic benefits in the intestine due to the anti-inflammatory properties of L. rhamnosus GR-1 and L. reuteri RC-14 (53).
Probiotics for vaginal and bladder health
In the 1930s, L. casei Shirota (Yakult) was first used commercially in Japan, then L. acidophilus NCFM (mid-1970s) was studied extensively for the gut health (54) and L. rhamnosus GR-1 and L. fermentum RC-14 were used for the urogenital tract [1980–1985] (55). After that, multiple attempts were made to ascertain appropriate probiotic agents for preventing vaginal colonization (56-58).
The positive results from in vitro studies may not be sufficient to envisage the competence of probiotics in humans. The diseases associated with absence of Lactobacilli do not really mean that administration of Lactobacilli to the vagina will stop or cure them. Since it is important that the strain adheres, colonizes for an adequate period of time for days or even weeks to confer health benefits to the host (13,59). Conversely, long-term colonization for months or years may not be required if the individuals own Lactobacilli recolonize or the external therapy is repeated. Without paying much attention to these points, some researchers have tested Lactobacillus strains unsuitable to the vagina (60,16). So, the strains had little proven impact on the relapse of infection. Others had taken these products to market with nominal scientific confirmation of effectiveness of a tested strain (61). Such strains may be a useful treatment option and provide some relief of symptoms, but publication of supportive efficacy data would increase the approval and reliability of consumers and medical practitioners.
The treatment of BV and possibly UTI were often made by introducing Lactobacilli into the vagina via a capsule or pessary to boost the microflora or overcome the pathogens by reducing their ability to dominate (62-64); but there is little proof to advocate that Lactobacilli can cure yeast vaginitis (65,66). The dried powder of Lactobacilli used in vaginal suppositories appeared to be capable of rehydrating and inhibiting pathogenic microbes. Skim milk-based preparations were also proven to be useful (15). Oral dosage (109 viable bacteria) was required once or twice weekly, vaginal protocol might initially be required once daily for 3 days to dislocate huge pathogenic biofilms in the urogenital system (12).
Potential mechanism of action of probiotics
The surface areas of our body, those are exposed to external environment are colonized by the resident microflora. But this complex habitat comprises of epithelial cells that undergo regular desquamation, they have secreted immunoglobulin A (IgA), other gland secretions and layers of mucoproteins. Probiotics are often administered to establish and maintain the ecological equilibrium of healthy microflora as they can promote biofilm formation, produce antagonistic substances and protect against infectious agents. Figure 1 depicts the possible mechanisms of action of probiotics against invading pathogens present in intestine, skin, respiratory tract and female urogenital tract. The most important and effective mechanism through which probiotics inhibit urogenital infections and exercise their positive effects is due to their ability to block adhesion and entry of pathogen and/or control the multiplication of pathogens, which they attain by their inherent properties such as generation of several antimicrobial metabolites (lactic, acetic, butyric acids and H2O2) and release of a variety of bacteriocins (e.g., lactocin, acidocin); ability to compete for space (receptor binding sites on vaginal epithelium); nutrients; adhere to uroepithelial cells; inhibit bacterial toxin production thereby prevent or cure bacterial infections as well as to release collagen-binding protein that prevents the attachment of pathogens (26,67-70). Figure 2 represents the different biomolecules released by Lactobacilli—the most frequently used probiotic.
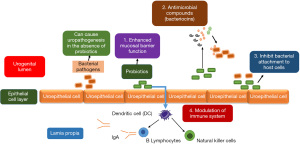

Probiotics can mend human health by oral consumption or local application. Probiotics especially Lactobacilli maintain the natural microbial balance beneficial to the host. They play an important role in modulating specific, non-specific, humoral and cellular responses of the human immune system by interacting with the mucosa-associated lymphoid tissue (MALT) (71-73). The mechanisms by which the probiotic bacteria influence the urogenital microflora and confer the desired effect in the urogenital tract is multifactorial, which are listed below under different subheadings.
Adherence to epithelial cells, colonization capabilities
Adhesion is the first step of pathogenesis and adherence to host mucosal surfaces is considered as an essential requirement for bacterial colonization and subsequent infection. Both native and pathogenic bacteria are capable to adhere to the epithelial host cells. Adherence is affected by the composition of mucosal surfaces. This adhesion to the vaginal mucosa is an essential and critical parameter for successful colonization of the microbiota and is carried out by two methods: specific adhesion involving bacterial adhesins and specific host epithelial receptors and nonspecific adhesion resulted due to interaction between bacteria and mucosa based on their physicochemical properties (74).
Biofilm formation
Biofilm formation was first reported for microbes of the oral cavity (75) and later specified in the urogenital tract (76). After bacterial adherence to the mucus layer or epithelial cell surface, they form a protective biofilm and their colonization is then promoted for establishment of persistent residents. The aggregation or coaggregation properties will help them to maintain a steady inhabitant, and they can also contribute for competing specific attachment sites by steric hindrance (competitive exclusion), thus evading attachment, growth or colonization of the pathogenic bacteria (77,78).
Production of biosurfactants
Biosurfactants are detergent molecules produced by certain microbes (79). Several physiological roles are ascribed to them as they interfere with surface tension (80). Biosurfactant producing bacteria can block pathogenic adhesion by acting as a competitive barrier (81). Lactobacillus is reported to produce biosurfactants, for example, surfactin is produced by L. acidophilus and L. fermentum strains (82). Surfactin molecule is reported to inhibit the adhesion of almost all the pathogens responsible for urogenital infections including E. coli, E. faecalis, and C. albicans. Biosurfactants play a vital role in biofilm establishment by probiotics and elimination of uropathogens from the urinary tract.
Aggregation and co-aggregation
Aggregation is the collaboration between two microorganisms belonging to same strain, while coaggregation is the interaction between the microbes of different strains or species. Both play important role in establishing a healthy microflora (83). In the vaginal environment, aggregation promotes biofilm development or the colonization of beneficial organisms (84). The coaggregation can happen between pathogens and Lactobacillus, permitting the pathogens to bind to the vaginal mucosa (85). This feature is genetically coded, since the genes liable for synthesis of an auto-aggregating factor of 32 kDa of L. gasseri 4B2 (86) were expressed more in exponential growth phase rather in stationary phase and a specific factor promoting aggregation (FPA) was detected in certain vaginal Lactobacillus strains, as in L. gasseri or L. coryniformis coaggregating E. coli and Campylobacter jejuni (87). The aggregation-promoting factor of L. gasseri 4B2 is transmissible as it was able to mediate a high frequency of conjugation between Lactobacillus strains (88). But the link between these features and the protection against urogenital infections are yet to be established.
Production of antagonistic substances
The beneficial properties of probiotic bacteria (Lactobacillus) are dependent on their metabolic activities and production of some antagonistic substances that play important role in protecting the urogenital tract are as follows:
Organic acids
Glycogen is the key carbon source at the surface of vaginal epithelium and its concentration varies with the hormonal cycle. The resident lactic acid bacteria and related microorganisms (Lactobacillus, Bifidobacterium, Streptococci), ferment glycogen or glucose to lactic acid (89,90), which lowers the pH of the vaginal tract (pH 4.5 or lower) and prevents the growth of most of the pathogens. But, disruption of ecological balance due to rise in numbers of other microbes (as in the case of BV) alters pH. Application of probiotic bacteria can increase the levels of lactic acid, impeding the growth of pathogenic entities (56). There are other short chain organic acids, like acetic or propionic acid, which are formed by heterolactic bacteria, also inhibit pathogens.
Hydrogen peroxide (H2O2)
H2O2 producing Lactobacillus plays an important role in balancing vaginal flora. L. crispatus and L. jensenii are found in 96% of healthy women and it is only 3.5% in women having BV (91). This characteristic is genetically determined, found only in some specific strains, as it was not made by all the vaginal isolates (56,91,92). H2O2 and its metabolites (OH−, O2−) are toxic to the cells as they attack and damage nucleic acids and proteins, leading to cellular death. The H2O2 produced by Lactobacillus constrains the growth of G. vaginalis, E. coli and S. aureus, inhibits HIV in vitro (93). Atopobium vaginae, anaerobic bacteria involved in BV is sensitive to H2O2.
Bacteriocin production
The production of bacteriocins by probiotics is also crucial in constraining urogenital infections. Bacteriocins are proteins synthesized by bacteria with constricted microbicidal activities (94). Molecules converted to bacteriocin-like metabolites executed broad range of microbicidal properties. They bind to specific receptor on target cells and create pores in their cell membranes, thereby disrupt the membrane transport. One bacteriocin-like substance isolated from L. salivarius strain was active against a wide variety of urogenital pathogens such as G. vaginalis, Enterococus faecalis, Neisseria gonorrhea (92).
Clinical studies
Mostly, urogenital flora originates from the gut and enters into the urogenital tract. It is evident that daily oral intake of L. rhamnosus GR-1 and L. fermentum RC-14 can amend the vaginal microflora (12,95). In a clinical trial, regular intake of L. rhamnosus GR-1 and L. fermentum RC-14 considerably reduced the number of coliforms and yeasts in the vagina (95). When blinded vaginal swabs were cultured, it was observed that significantly more number of Lactobacilli and less number of yeast and fewer coliforms were detected in the Lactobacilli-treated group; while the swabs acquired from placebo group exhibited a substantial escalation in yeast and coliforms. Probiotic administration regularized the flora in some cases of BV, suggesting this to be an effective and long-lasting therapy for pregnant women and those at risk of UTI and BV. These organisms were also found to displace other organisms in vitro as observed in another study (96).
Use of L. rhamnosus GG strain in fermented milk decreased UTI recurrences (97). The efficacy of intestinal probiotics to influence vaginal and bladder health through some kind of immunomodulation is not yet proven, but a study revealed that L. casei Shirota had prospective to moderate the recurrence of bladder cancer (49).
There were few investigations piloted before 1994 to assess the probiotic capability of Lactobacillus introduced by intravaginal method in women with UTI (15,59,60). No effect was observed on the occurrence of recurrent infection (60). In contrast, Reid et al. [1992] reported 21% of recurrences in the treated group using a well characterized strain versus 47% in the placebo group (98). These authors published a clinical trial in 1995 and found that oral administration was ineffective in decreasing the frequency of infection (63). The effectiveness of oral administration of L. rhamnosus GR-1 and L. fermentum RC-14 was tested in another study and it was found that these organisms were irregularly collected from the vagina (12) and a drink comprising L. rhamnosus GG was unsuccessful in decreasing the rate of recurrent UTI (16). The efficacy of probiotics in BV remains poorly studied. There was a decreased clinical cure rate for BV treated with L. acidophilus suppositories (99). Consumption of yoghurt containing L. acidophilus reduced the incidence of BV (66). Daily oral administration of L. rhamnosus GR-1 and L. fermentum RC-14 restored normal Lactobacilli flora in asymptomatic BV (95). L. crispatus CTV05 was tested for prevention of recurrences of BV (14). Though Lactobacillus is detected in majority of vaginal candidiasis it does not eliminate the prophylactic role of certain strains in VVC. Clinical studies were conducted to elucidate this protective role, by using Lactobacilli in the form of vaginal suppositories or yoghurt (65,66). Regrettably, some of these studies were carried out with poorly characterized strains. Consumption of yoghurt with Lactobacillus was unable to diminish the frequency of symptomatic vulvovaginitis or Candida infection (66). Reduction in Candida colonization was observed in patients supplied with Lactobacillus containing yoghurt than in the placebo group (100). When a patient with recurrent vulvovaginitis was treated with the L. rhamnosus GR-1 strain, recolonization of the vaginal epithelium and a 6-month symptom-free period was observed (59). In a pilot two-patient study, the management of UTI in patients with SCI was assessed. L. rhamnosus GR-1 and L. reuteri were supplied to one patient and placebo to other along with antibiotics to cure acute UTI. Urinary TNF-alpha was noticeably down regulated in the individual who used intermittent catheterization and received the probiotic in comparison to the individual who had indwelling catheter and received placebo (101).
The effectiveness of a single or cocktail strain of probiotics supplied orally or intravaginally for treating BV was explored (22). Two types of experimental designs were used for treatment of BV, i.e., first, BV therapy was carried out with only probiotics; second, probiotics were given after antibiotic therapy. A combination of different species of Lactobacilli with different biological properties, i.e., L. rhamnosus GR-1 and L. fermentum RC-14 were used on fertile nonpregnant women for treatment of genitourinary infections (50,102). In a placebo-controlled study, a better BV cure rate of 88% was detected by using a pharmaceutical product (comprising a H2O2-producing L. acidophilus strain plus estriol) that involved both pregnant and non-pregnant women (103). A similar product with H2O2-producing L. acidophilus was proven to be unsuccessful for curing BV as evaluated according to Amsel criteria (99). Nevertheless, the findings of the study could not be accepted as 50% of the patients in the active group and 86% of the placebo group withdraw from the trial.
When antimicrobial metronidazole therapy for BV was supplemented with a 30-day oral probiotic treatment (L. rhamnosus GR-1 and L. fermentum RC-14) and compared to placebo-treated control, it was found that towards the end of the procedure, a great number of women in the probiotic group were BV-free as compared to the control group (Nugent score ≤3) (50). The effect of Lactobacillus augmentation after metronidazole or clindamycin therapy on the recurrence of BV was assessed in few trials (104,105). Probiotic use considerably decreased the recurrence rate of BV at 6 months from beginning of the procedure. Use of tampons saturated with L. gasseri, L. casei subsp. rhamnosus and L. fermentum or placebo tampons during the menstrual period following clindamycin treatment were explored (104). There were no significant differences between the two groups possibly due to low count of Lactobacilli in tampons at the end of the study (106 PFU) or the unfavorable period of administration, i.e., during the menstrual flow. In another study, the efficacy of vaginal probiotic capsules for BV prophylaxis in healthy women with a history of recurrent BV was evaluated. One hundred and twenty healthy Chinese women with recurrent BV (≥2 BV episodes in the preceding year) were dispensed arbitrarily for vaginal prophylaxis with daily 1 capsule containing L. rhamnosus [6.8×109 colony-forming unit (CFU)], L. acidophilus (0.4×109 CFU) and Streptococcus thermophiles (0.4×109 CFU) or 1 placebo capsule for 7 days on, 7 days off, and 7 days on. Probiotic treatment ensued lower recurrence of BV (15.8% vs. 45.0%; P<0.001). During the 2- and 11-month sequel period, probiotics group had lower incidence of BV (10.6% vs. 27.7%; P=0.04). The major drawback of the study was that 11-month results were gathered through telephonic interview, but not through physical interview (106).
Vaginal infections and probiotic preparations
Changes in the composition of the vaginal flora, particularly the partial or complete depletion of Lactobacilli in cases of vaginal and lower UTIs, highlight the potential for using probiotic preparations to restore the balance of the natural vaginal microflora and thereby preventing the invasion and controlling the growth of pathogens. The rationale for this approach, based on the outcome of clinical trials, is quite strong (63,95,107,108), although there are still a number of discrepancies among the reported results. They can be attributed to the wide range of products used, with varying concentrations, viabilities and properties of the bacteria administered (68,70,107). Over previous decades, mostly anecdotal reports were published in which promising results with oral or local applications of yoghurt or Lactobacilli cultures for the treatment of vaginosis and vaginitis were reported (26,67,70,109). Although they showed a significant eradication of pathogens, reporting a success rate of up to 54%, they were conducted without randomization or satisfactory controls (110).
More recently, placebo-controlled, cross-over trials performed by Hilton and collaborators using L. acidophilus-containing yoghurt had a cure rate ranging from 57% for BV to 74% for yeast vaginitis, compared with a cure rate of 0–22% observed in the control group (65). Israeli researchers using L. acidophilus-containing yoghurt found a decrease in the episodes of BV among the participants ingesting it, but did not observe a significant effect of this treatment on the occurrence of Candida vaginitis (66). However, the authors did observe a decrease in the number of Candida-positive vaginal cultures among all participants consuming both types of yoghurt (i.e., those consuming Lactobacilli-enriched and “plain” yoghurt), from 60% during the first month to 28% during the second (66).
In randomized, placebo-controlled and double-blind trials conducted by Hallén and co-workers lyophilized preparations of L. acidophilus applied locally (pessaries) had a success rate of 57% in the probiotic-treated group versus 0% recovery in the control group (99). Similarly in a randomized, placebo controlled multi-centre study, Parent’s group reported a cure rate of 88% for the probiotic-treated group and 22% for the placebo group, using a local application of lyophilized preparation of H2O2-producing L. acidophilus (103). Furthermore, a double-blind, placebo-controlled study was conducted to evaluate the potential methods of self-treatment among HIV-positive women over a period of 21 months. Williams et al. [2001] found that the locally applied L. acidophilus preparations were effective in the prevention of VVC (111).
Lower UTIs and probiotic preparations
The efficacy of probiotic preparations in the treatment of lower UTIs and/or disorders has also been investigated in a number of studies. A double-blind trial conducted on 138 patients with superficial transitional cell carcinoma of the bladder indicated that L. casei had a preventative effect on the recurrence of superficial bladder cancer (49). On the other hand, in a double-blind study involving 585 preterm infants, although UTIs and necrotizing enterocolitis were found less frequently in the Lactobacillus GG treated group, the differences were not statistically significant (112). It is possible that if the probiotic treatment were to be based on the application of Bifidobacterium infantis, which constitutes the dominant microflora of healthy, breast-fed infants (113), the outcome could be different. The clinical trials on the use of probiotics were compiled in Table 4.
Table 4
| Name of the probiotics | Dose & mode of administration | Study design | Results | Reference |
|---|---|---|---|---|
| Lactobacillus rhamnosus GR-1 ve Lactobacillus reuteri B-54 | Vaginal, 1×109 CFU/L. rhamnosus GR-1 ve L. fermentum B-54 | 55 pre-menopausal women; randomized double-blind study | Recurrence rate was 73% in 25 patients who received weekly one dose of vaginal ovules containing L. rhamnosus GR-1 ve L. reuteri B-54; 79% decrease was observed in recurrence rates in 30 patients who received once weekly doses of intravaginal Lactobacilli growth factor | (63) |
| L. rhamnosus GR-1 and L. fermentum RC-14 | Not specified | Randomized, double-blind, placebo-controlled study of 64 healthy women | Significantly less yeast and fewer coliforms in the vagina | (95) |
| Lactobacillus ovules | Vaginal, 108 CFUs/mL L. crispatus | Randomized placebo-controlled, double-blind study including 100 premenopausal women | Vaginal ovule containing L. crispatus, and placebo were applied for 10 weeks, and a significant decrease in disease was observed in the probiotic group | (46) |
| Antimicrobial treatment and Lactobacillus ovules | Vaginal, >1.6×109 CFU/L. rhamnosus GR-1 and L. fermentum B-54 | Randomized placebo-controlled, double-blind study including 41 pre-, and postmenopausal patients | Recurrence rate: 29% in UTI patients receiving antimicrobial treatment | (98) |
| Recurrence rate was observed to be 21% in patients who received vaginal ovules containing Lactobacillus spp. following antimicrobial treatment | ||||
| Lactobacillus drink, and fruit juice | Oral, 4×1010 CFU/100 L. rhamnosus GG | 324 pre-menopausal patients, randomized placebo-controlled double-blind study | The risk of UTI decreased significantly using fruit juice containing probiotic in 139 patients diagnosed with acute UTI | (97) |
| Lactobacillus sp. | Vaginal suppositories, post therapy, randomly treated for 3 days with norfloxacin or trimethoprim/sulfamethoxazole (TMP/SMX) | 41 adult women with acute lower UTIs | 21% of recurrences in the treated group using a well characterized strain versus 47% in the placebo group | (98) |
| Sterilized skim-milk suppositories | ||||
| L. rhamnosus gR-1, L. reuteri RC-14 | Daily vaginal capsule containing 65% compared to 33% L. rhamnosus gR-1 (109 CFU) metronidazole and L. reuteri RC-14 (109 CFU) or 0.75% metronidazole gel, b.i.d. for 5 days | Randomized, observer blind, active controlled 30 days | 65% compared to 33% metronidazole (P=0.056) | (50) |
| L. brevis CD2, L. salivarius FV2, and L. plantarum FV9 | Daily vaginal tablet containing ≥109 CFU of L. brevis CD2, L. salivarius FV2, and L. plantarum FV9 for 7 days | Randomized, double blind, placebo controlled, 3 weeks | 50% compared to 6% control (P=0.017) | (114) |
| L. acidophilus | Vaginal suppository containing L. acidophilus 108–109 CFU or placebo control, b.i.d. for 6 days | Randomized, double blind, placebo controlled, 20–40 days | 21% compared to 0% control (P = NS) | (99) |
| L. acidophilus | 1–2 daily vaginal tablet containing L. acidophilus ≥107 CFU and 0.03 mg control, estriol for 6 days | Randomized, placebo controlled 4 weeks | 88% compared to 22% control (P<0.05) | (103) |
| L. casei rhamnosus | Oral clindamycin 300 mg b.i.d. for 7 days, then vaginal capsules containing 109 CFU of L. casei rhamnosus for 7 days | Randomized, observer blind placebo controlled 4 weeks | 83% compared to 35% control (P<0.001) | (115) |
| L. gasseri Lba, L. rhamnosus Lbp | Vaginal 2% clindamycin cream directly followed 6 menstrual by vaginal capsules containing L. gasseri Lba periods EB01-DSM 14869 (108–109 CFU) and L. rhamnosus Lbp PB01-DSM 14870 (108–109 CFU) for 10 days, probiotic treatment repeated for 10 days after each menstruation during 3 menstrual cycles | Randomized, double blind, placebo controlled 6 menstrual periods | 65% compared to 46% control (P=0.042) | (116) |
| L. gasseri, L. casei rhamnosus, L. fermentum | Vaginal 100 mg clindamycin ovules for 3 days, 2 menstrual then tampons containing 108 CFU of L. gasseri, periods L. casei rhamnosus, L. fermentum or placebo tampons during the next menstrual period | Randomized, double blind, placebo controlled 2 menstrual periods | 56% compared to 62% control (P = NS) | (104) |
| L. acidophilus | Oral metronidazole 400 mg b.i.d. for 7 days followed by vaginal pessary containing L. acidophilus KS400 ≥107 CFU and 0.03 mg estriol for 12 days | 6 months | 72% compared to 73% control (P = NS) | (105) |
CFU, colony-forming unit; NS, not significant; UTI, urinary tract infection.
In vitro studies
The vaginal microflora protects it from assaulting pathogens comprising those that cause UTIs and sexually transmitted diseases. The potency of Lactobacilli as probiotics was tested for maintaining urogenital tract well-being and prevention or cure of urogenital infections. L. rhamnosus L60 isolated from the vagina of healthy, nonpregnant, premenopausal women was assessed for its in vitro antimicrobial activity, antibiotic resistance, H2O2 production, auto-aggregation, co-aggregation with other bacterial species, bacterial adherence and surface hydrophobicity. It presented resistance to antibiotics commonly used against uropathogens and broad spectrum of antimicrobial activity against urogenital pathogens. L60 produced H2O2, displayed self-aggregation, adhered to vaginal epithelial cells and co-aggregated with E. coli and C. albicans. Based on these notes, L60 is considered as a potential probiotic (117). Certain Lactobacillus strains blocked adhesion of yeasts to the vaginal epithelium and secreted substances that block their growth (57).
The antagonistic activity and anti-oxidative potential of five probiotic Lactobacilli (L. rhamnosus GG, L. plantarum 299v, L. acidophilus La5, L. paracasei 8700:2 and L. fermentum ME-3) and two Bifidobacteria (B. lactis Bb12, B. longum 46) against six pathogenic bacteria was assessed by following different methods (anaerobic and microaerobic culture; solid or liquid media). Pyelonephritic E. coli was greatly repressed by GG and both Bifidobacteria strains. Lactobacilli strains ME-3, 299v and 8700:2 were most potential against different enteropathogens. So, these probiotics could be effective in treating GI and UTIs (19).
UTI can be cured or prevented by abrogation of biofilm formation. The efficacy of the cell free supernatant (CFS) of Lactobacillus strains purified from kefir were evaluated for their antibacterial and antibiofilm efficacies against uropathogenic E. coli (UPEC). All the purified strains displayed co-aggregation property with E. coli. Auto-aggregation was highly correlated with co-aggregation of all Lactobacilli strains with E. coli and L. rhamnosus and L. paracasei showed maximum antibiofilm and bactericidal activity (118).
In vivo studies
Lactobacilli prevented UTI inception in animal models and restored normal vaginal flora (119). In a mouse animal model, intraurethral introduction of L. fermentum CRL 1058 resulted no opposing effects or substantial variations in the kidney, ureter, bladder or urethra. Therefore, it was concluded that Lactobacilli can be used safely for probiotic therapy (20). The antimicrobial activity of the intraurethrally administered probiotic L. casei strain Shirota was tested against E. coli in a mouse UTI model. A chronic infection was retained for over 3 weeks in the urinary tract (bladder and kidneys) by 106 CFU pathogens after the challenge. The myeloperoxidase activity and polymorphonuclear leukocyte number in the urine were significantly raised during the period of infection. Application of one dose of L. casei Shirota (108 CFU), 24 h before the trial infection vividly repressed E. coli growth and inflammatory responses in the urinary tract. Multiple daily treatments with L. casei Shirota during the post infection period exhibited substantial antibacterial activity, even a heat-killed formulation of L. casei Shirota presented noted antimicrobial activity with single or multiple daily treatments during the post infection period. The other Lactobacillus strains tested, i.e., L. fermentum ATCC14931T, L. plantarum ATCC14917T, L. reuteri JCM 1112T and L. jensenii ATCC25258T had no significant antimicrobial activity. Therefore, these results propose that the probiotic L. casei strain Shirota is a potent therapeutic agent for UTI (120).
A prospective, controlled study was undertaken with 35 healthy, sterilized bitches without any past account of recurrent UTIs. Vaginal swab culture was collected from each dog before and after oral probiotic supplementation. Twenty-three dogs received probiotic supplement daily for 14 days and 12 dogs were given the supplement daily for 28 days. Lactic acid-producing bacteria (LAB) were isolated from 7 out of 35 dogs before probiotic receipt. Oral probiotic administration for 14 or 28 days did not alter the occurrence of vaginal LAB in dogs (121).
Issues/problems relevant to marketing of probiotics
Strict regulation and stringent guidelines should be there on the usage and marketing of probiotics because many of the alleged probiotic products presently on the market have never been scientifically proven, using suitable methods to prove their health benefits; in fact, many of them had either different strains that are mentioned on the label or ineffective dead organisms (122-125). Moreover, some unreliable marketing companies make bizarre and wrong claims in their publicity material, including content sited on their web sites (12). As an outcome, the probiotics therapy concept had lost confidence among the Medicare providers and sometimes the consumers bought and used erratic products, which never fulfilled their desired outcomes. Therefore, rigorous clinical studies and extensive education about probiotics prompted by good science is required to change the perceptions of medical community about probiotics.
Discussion
The health benefits of probiotics were first documented for gastro-intestinal tract problems, but the findings are not limited to only GI tract infections or ailments rather there is increasing evidences that these benefits can extend to the urogenital tract where these preparations restore the natural balance of the indigenous residents and possibly inhibit the entry of a broad array of pathogens. A number of researchers found that orally or vaginally administered probiotic preparations were able to inhabit the epithelium of vagina. After their establishment, they can protect the urogenital tract by preventing and inhibiting infectious agents.
Unlike antibiotics, probiotics can be used for a longer duration without any antagonistic side effects. Most of the clinical trials with better results were achieved by using greater doses of Lactobacilli (around 109 CFU), which suggests that, along with good strain characteristics, the amount of exogenously applied Lactobacilli is also important for its effectiveness and their prolonged use is also more encouraging than short courses (105,106). The desired route of delivery for probiotic Lactobacilli is intravaginal. But some workers also tried Lactobacilli via oral route to re-inhabit the vagina, based on the reports that pathogens can enter the urogenital tract from the gut and Lactobacillus strains were recovered from the vagina after oral administration (126). But the capacity of the Lactobacilli to inhabit the vagina after oral ingestion is firmly reliant on their sustainability and potentiality to survive gastric acid and bile salts. Moreover, the Lactobacilli that reach the vagina may not bring the expected outcome as the gut flora and the vaginal flora vary significantly, which eliminates the direct entry of all the species and strains present in the gut into the urogenital tract. Since none of the trials had estimated the vaginal colonization of oral Lactobacilli in BV, it is possible that the bacteria might have exercised a systemic immunomodulating activity thereby improving the conditions. Apparently, the time required for vaginal colonization after oral supplementation is much slower as compared to direct vaginal application. In addition, the load of Lactobacilli that can be delivered orally to the vagina is clearly lower than direct vaginal administration. Therefore, its effectiveness also would be lower in comparison to direct vaginal application.
Numerous experiments have been conducted to examine the efficacy of different strains of Lactobacilli, received either orally or intra-vaginally, along with/without antibiotics, could be beneficial in the prophylaxis or treatment of vaginal infections. Although the species used in the various trials were different, 3 out of 4 studies reported a significant cure rate (50,102,103). Once probiotics were used after antibiotic therapy, the BV recovery rate was increased significantly and recurrence rates were reduced appreciably in 3 out of 5 studies (50,115,116). Regardless of the key opposing illnesses allied with atypical vaginal flora, no protective treatments are available till date. The only clinical trial with positive results were obtained in healthy Chinese women by using Lactobacilli (106). These results support the potential use of probiotics for BV treatment and cure. However, more evidences are needed to establish these claims, as extensive heterogeneity in probiotic products, trial protocols and outcome measures do not supply adequate data for or against endorsing probiotics for BV therapy. Moreover, these studies with probiotics for BV have been piloted by using diverse population of bacterial species and strains, route of administration, dose schedule and duration of treatment under a particular study. All of these differences could act as unclear factors impeding an actual evaluation among the trials and also may account for the dissimilar outcomes of the treatments. Therefore, well-planned randomized controlled trials with uniform approaches are required to approve the profits of probiotics in the treatment of BV and other urological ailments.
Conclusion & future perspectives
Urological distresses are most frequently experienced, discomforting problems and probiotics are gaining popularity to be used for improving our overall immune status because of their immense benign and beneficial effects. Their therapeutic and curative efficacies are proven in many experiments. Scientific evidences indicating that probiotics have considerable therapeutic value are also accumulating. Therefore, this narrative overview is prepared on probiotics, which could be beneficial for general public, medical practitioners, entrepreneurs and policy makers, who are looking for evidence based alternative remedies for urinary and other relevant disorders. On the basis of the evidences presented in this review, it could be concluded that probiotic prophylaxis and therapy can be availed to reduce the chances and recurrences of vaginosis, vaginitis, lower UTIs and possibly in other urological conditions.
Acknowledgments
The authors thank Dr. Noor Bucholtz for being instrumental to encourage the authors to work on this subject and prepare this review article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Noor N. P. Buchholz) for the series “Integrative Medicine Approaches to Common Urological Problems” published in Longhua Chinese Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-62/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-62/coif). The series “Integrative Medicine Approaches to Common Urological Problems” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Metchnikoff E. The prolongation of life: Optimistic studies. London: G. P. Putnam & Sons, 1907.
- FAO/WHO, author. Guidelines for the evaluation of probiotics in food. Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report. 2002. Available online: http://www.fao.org/es/ESN/Probio/probio.htm_2002
- Shewale RN, Sawale PD, Khedkar CD, et al. Selection criteria for probiotics: a review. Int J Probiotics Prebiotics 2014;9:17-22.
- Bengmark S. Gut microbial ecology in critical illness: is there a role for prebiotics, probiotics, and synbiotics? Curr Opin Crit Care 2002;8:145-51. [Crossref] [PubMed]
- Fuller R. Probiotics in man and animals. J Appl Bacteriol 1989;66:365-78. [Crossref] [PubMed]
- Singh VP, Sharma J, Babu S, et al. Role of probiotics in health and disease: a review. J Pak Med Assoc 2013;63:253-7. [PubMed]
- Available online: https://www.healthline.com/health/renal-and-urological-disorders#types
- Available online: https://www.urologyhealth.org/urologic-conditions/urinary-tract-infections-in-adults
- Available online: https://healthblog.uofmhealth.org/womens-health/a-female-urologist-explains-womens-most-common-urological-concerns-and-how-to-treat
- Available online: https://www.mydailynews.com/life-style/8-common-urological-issues-affecting-men-article-1.3594438
- Reid G. The scientific basis for probiotic strains of Lactobacillus. Appl Environ Microbiol 1999;65:3763-6. [Crossref] [PubMed]
- Reid G, Bruce AW. Selection of lactobacillus strains for urogenital probiotic applications. J Infect Dis 2001;183:S77-80. [Crossref] [PubMed]
- Cadieux P, Burton J, Gardiner G, et al. Lactobacillus strains and vaginal ecology. JAMA 2002;287:1940-1. [Crossref] [PubMed]
- Antonio MA, Hillier SL. DNA fingerprinting of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J Clin Microbiol 2003;41:1881-7. [Crossref] [PubMed]
- Bruce AW, Reid G. Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can J Microbiol 1988;34:339-43. [Crossref] [PubMed]
- Kontiokari T, Sundqvist K, Nuutinen M, et al. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 2001;322:1571. [Crossref] [PubMed]
- Bertuccini L, Russo R, Iosi F, et al. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int J Immunopathol Pharmacol 2017;30:163-7. [Crossref] [PubMed]
- Lee SJ, Shim YH, Cho SJ, et al. Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatr Nephrol 2007;22:1315-20. [Crossref] [PubMed]
- Hütt P, Shchepetova J, Lõivukene K, et al. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol 2006;100:1324-32. [Crossref] [PubMed]
- Silva de Ruiz C. del R Rey M, Nader-Macías ME. Structural and ultrastructural studies of the urinary tract of mice inoculated with Lactobacillus fermentum. BJU Int 2003;91:878-82. [Crossref] [PubMed]
- Torzewska A, Wiewiura P, Brodecka D, et al. Potentially Probiotic Lactobacillus Strains Derived from Food Intensify Crystallization Caused by Proteus mirabilis in Urine. Probiotics Antimicrob Proteins 2021;13:441-52. [Crossref] [PubMed]
- Falagas M, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect 2007;13:657-64. [Crossref] [PubMed]
- Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005;353:1899-911. [Crossref] [PubMed]
- Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev 2010;23:253-73. [Crossref] [PubMed]
- Köhler GA, Assefa S, Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol 2012;2012:636474. [Crossref] [PubMed]
- Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr 1999;39:13-126. [Crossref] [PubMed]
- Salminen S, Arvilommi H. Probiotics demonstrating efficacy in clinical settings. Clin Infect Dis 2001;32:1577-8. [Crossref] [PubMed]
- Alvarez-Olmos MI, Oberhelman RA. Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clin Infect Dis 2001;32:1567-76. [Crossref] [PubMed]
- Salminen MK, Tynkkynen S, Rautelin H, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis 2002;35:1155-60. [Crossref] [PubMed]
- Sipsas NV, Zonios DI, Kordossis T. Safety of Lactobacillus strains used as probiotic agents. Clin Infect Dis 2002;34:1283-4; author reply 1284-5. [Crossref] [PubMed]
- Stapleton A. Novel approaches to prevention of urinary tract infections. Infect Dis Clin North Am 2003;17:457-71. [Crossref] [PubMed]
- Reid G, Bruce AW. Urogenital infections in women: can probiotics help? Postgrad Med J 2003;79:428-32. [Crossref] [PubMed]
- Farage MA, Miller KW, Sobel JD. Dynamics of the vaginal ecosystem—hormonal influences. Infectious Diseases: Research and Treatment 2010;3:1-15.
- Sieber R, Dietz UT. Lactobacillus acidophilus and Yogurt in the Prevention and Therapy of Bacterial Vaginosis. International Dairy Journal 1998;8:599-607. [Crossref]
- Waigankar SS, Patel V. Role of probiotics in urogenital healthcare. J Midlife Health 2011;2:5-10. [Crossref] [PubMed]
- Bik EM, Bir SW, Bustamante JP, et al. A novel sequencing-based vaginal health assay combining self-sampling, HPV detection and genotyping, STI detection, and vaginal microbiome analysis. PLoS One 2019;14:e0215945. [Crossref] [PubMed]
- Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis 1999;180:1950-6. [Crossref] [PubMed]
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother 2006;58:266-72. [Crossref] [PubMed]
- Reid G, Burton J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect 2002;4:319-24. [Crossref] [PubMed]
- Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther 2008;30:453-68. [Crossref] [PubMed]
- Cribby S, Taylor M, Reid G. Vaginal microbiota and the use of probiotics. Interdiscip Perspect Infect Dis 2008;2008:256490. [Crossref] [PubMed]
- Livengood CH 3rd, Thomason JL, Hill GB. Bacterial vaginosis: diagnostic and pathogenetic findings during topical clindamycin therapy. Am J Obstet Gynecol 1990;163:515-20. [Crossref] [PubMed]
- Menard JP. Antibacterial treatment of bacterial vaginosis: current and emerging therapies. Int J Womens Health 2011;3:295-305. [Crossref] [PubMed]
- Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med 1995;25:587-91. [Crossref] [PubMed]
- Zeng ZM, Liao QP, Yao C, et al. Directed shift of vaginal flora after topical application of sucrose gel in a phase III clinical trial: a novel treatment for bacterial vaginosis. Chin Med J (Engl) 2010;123:2051-7. [PubMed]
- Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 2011;52:1212-7. [Crossref] [PubMed]
- Gille C, Böer B, Marschal M, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol 2016;215:608.e1-7. [Crossref] [PubMed]
- Wall BM, Dmochowski RR, Malecha M, et al. Inducible nitric oxide synthase in the bladder of spinal cord injured patients with a chronic indwelling urinary catheter. J Urol 2001;165:1457-61. [Crossref] [PubMed]
- Aso Y, Akaza H, Kotake T, et al. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol 1995;27:104-9. [Crossref] [PubMed]
- Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect 2006;8:1450-4. [Crossref] [PubMed]
- Hagberg L, Bruce AW, Reid G et al. Colonization of the urinary tract with live bacteria from the normal fecal and urethral flora in patients with recurrent symptomatic urinary tract infections. In: Kass EH, Svanborg Eden C. editors. Host-Parasite Interactions in Urinary Tract Infections. Chicago, IL, USA: University of Chicago Press, 1989:194-7.
- Darouiche RO, Hull RA. Bacterial interference for prevention of urinary tract infection: an overview. J Spinal Cord Med 2000;23:136-41. [Crossref] [PubMed]
- Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 2007;13:35-7. [Crossref] [PubMed]
- Sanders ME, Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci 2001;84:319-31. [Crossref] [PubMed]
- Reid G, Cook RL, Bruce AW. Examination of strains of lactobacilli for properties that may influence bacterial interference in the urinary tract. J Urol 1987;138:330-5. [Crossref] [PubMed]
- Tomás MS, Bru E, Nader-Macías ME. Comparison of the growth and hydrogen peroxide production by vaginal probiotic lactobacilli under different culture conditions. Am J Obstet Gynecol 2003;188:35-44. [Crossref] [PubMed]
- Osset J, Bartolomé RM, García E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis 2001;183:485-91. [Crossref] [PubMed]
- Andreu A, Stapleton AE, Fennell CL, et al. Hemagglutination, adherence, and surface properties of vaginal Lactobacillus species. J Infect Dis 1995;171:1237-43. [Crossref] [PubMed]
- Reid G, Millsap K, Bruce AW. Implantation of Lactobacillus casei var rhamnosus into vagina. Lancet 1994;344:1229. [Crossref] [PubMed]
- Baerheim A, Larsen E, Digranes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scand J Prim Health Care 1994;12:239-43. [Crossref] [PubMed]
- Chlebecek J, Reich I. Fermalac Vaginal (Rougier Inc.) in the prevention of colpitis in pregnancy. Cesk Gynekol 1993;58:237-8. [PubMed]
- Burton JP, Reid G. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J Infect Dis 2002;186:1770-80. [Crossref] [PubMed]
- Reid G, Bruce AW, Taylor M. Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecol Ther 1995;23:32-45.
- Gardiner GE, Heinemann C, Bruce AW, et al. Persistence of Lactobacillus fermentum RC-14 and Lactobacillus rhamnosus GR-1 but Not L. rhamnosus GG in the Human Vagina as Demonstrated by Randomly Amplified Polymorphic DNA. Clin Diagn Lab Immunol 2002;9:92-6. [PubMed]
- Hilton E, Isenberg HD, Alperstein P, et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med 1992;116:353-7. [Crossref] [PubMed]
- Shalev E, Battino S, Weiner E, et al. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med 1996;5:593-6. [Crossref] [PubMed]
- Elmer GW, Surawicz CM, McFarland LV. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA 1996;275:870-6. [Crossref] [PubMed]
- Reid G, Charbonneau D, Erb J, et al. Ability of Lactobacillus GR-1 and RC-14 to stimulate host defenes and reduce gut translocation and infectivity of Salmonella typhimurium. Nutraceut Food 2002;7:168-73.
- Johannsen E. Probiotic bacteria: their properties and mode of action (Part 2 of 4). SA Fam Pract 2003;45:36-8.
- Jeavons HS. Prevention and treatment of vulvovaginal candidiasis using exogenous Lactobacillus. J Obstet Gynecol Neonatal Nurs 2003;32:287-96. [Crossref] [PubMed]
- Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr 2000;130:403S-9S. [Crossref] [PubMed]
- Isolauri E, Sütas Y, Kankaanpää P, et al. Probiotics: effects on immunity. Am J Clin Nutr 2001;73:444S-50S. [Crossref] [PubMed]
- Johannsen E, Viljoen M. Probiotics and the immune system. SAJNM 2001;5:66.
- Vesterlund S, Paltta J, Karp M, et al. Measurement of bacterial adhesion-in vitro evaluation of different methods. J Microbiol Methods 2005;60:225-33. [Crossref] [PubMed]
- Rickard AH, Gilbert P, High NJ, et al. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol 2003;11:94-100. [Crossref] [PubMed]
- Velraeds MM, van de Belt-Gritter B, Busscher HJ, et al. Inhibition of uropathogenic biofilm growth on silicone rubber in human urine by lactobacilli--a teleologic approach. World J Urol 2000;18:422-6. [Crossref] [PubMed]
- Ocaña VS, Nader-Macías ME. Vaginal lactobacilli: self- and co-aggregating ability. Br J Biomed Sci 2002;59:183-90. [Crossref] [PubMed]
- Juárez Tomás MS, Wiese B, Nader-Macías ME. Effects of culture conditions on the growth and auto-aggregation ability of vaginal Lactobacillus johnsonii CRL 1294. J Appl Microbiol 2005;99:1383-91. [Crossref] [PubMed]
- Velraeds MM, van der Mei HC, Reid G, et al. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol 1996;62:1958-63. [Crossref] [PubMed]
- Velraeds MM, van de Belt-Gritter B, van der Mei HC, et al. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. J Med Microbiol 1998;47:1081-5. [Crossref] [PubMed]
- Velraeds MM, van der Mei HC, Reid G, et al. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis to solid substrata by an adsorbed biosurfactant layer from Lactobacillus acidophilus. Urology 1997;49:790-4. [Crossref] [PubMed]
- Reid G, Heinemann C, Velraeds M, et al. Biosurfactants produced by Lactobacillus. Methods Enzymol 1999;310:426-33. [Crossref] [PubMed]
- Jankovic I, Ventura M, Meylan V, et al. Contribution of aggregation-promoting factor to maintenance of cell shape in Lactobacillus gasseri 4B2. J Bacteriol 2003;185:3288-96. [Crossref] [PubMed]
- Cesena C, Morelli L, Alander M, et al. Lactobacillus crispatus and its nonaggregating mutant in human colonization trials. J Dairy Sci 2001;84:1001-10. [Crossref] [PubMed]
- Kmet V, Lucchini F. Aggregation-promoting factor in human vaginal Lactobacillus strains. FEMS Immunol Med Microbiol 1997;19:111-4. [Crossref] [PubMed]
- Ventura M, Jankovic I, Walker DC, et al. Identification and characterization of novel surface proteins in Lactobacillus johnsonii and Lactobacillus gasseri. Appl Environ Microbiol 2002;68:6172-81. [Crossref] [PubMed]
- Schachtsiek M, Hammes WP, Hertel C. Characterization of Lactobacillus coryniformis DSM 20001T surface protein Cpf mediating coaggregation with and aggregation among pathogens. Appl Environ Microbiol 2004;70:7078-85. [Crossref] [PubMed]
- Reniero R, Cocconcelli P, Bottazzi V, et al. High frequency of conjugation in Lactobacillus mediated by an aggregation-promoting factor. J Gen Microbiol 1992;138:763-8. [Crossref]
- Boskey ER, Telsch KM, Whaley KJ, et al. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun 1999;67:5170-5. [Crossref] [PubMed]
- Boskey ER, Cone RA, Whaley KJ, et al. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod 2001;16:1809-13. [Crossref] [PubMed]
- Eschenbach DA, Davick PR, Williams BL, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 1989;27:251-6. [Crossref] [PubMed]
- Ocaña VS, de Ruiz Holgado AA, Nader-Macías ME. Growth inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus paracasei subsp. paracasei isolated from the human vagina. FEMS Immunol Med Microbiol 1999;23:87-92. [Crossref] [PubMed]
- Sha BE, Zariffard MR, Wang QJ, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis 2005;191:25-32. [Crossref] [PubMed]
- Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev 1995;59:171-200. [Crossref] [PubMed]
- Reid G, Bocking A. The potential for probiotics to prevent bacterial vaginosis and preterm labor. Am J Obstet Gynecol 2003;189:1202-8. [Crossref] [PubMed]
- Reid G, Tieszer C. Preferential adhesion of bacteria from a mixed population to a urinary catheter. Cells and Materials 1993;3:171-6.
- Kontiokari T, Laitinen J, Järvi L, et al. Dietary factors protecting women from urinary tract infection. Am J Clin Nutr 2003;77:600-4. [Crossref] [PubMed]
- Reid G, Bruce AW, Taylor M. Influence of three-day antimicrobial therapy and lactobacillus vaginal suppositories on recurrence of urinary tract infections. Clin Ther 1992;14:11-6. [PubMed]
- Hallén A, Jarstrand C, Påhlson C. Treatment of bacterial vaginosis with lactobacilli. Sex Transm Dis 1992;19:146-8. [Crossref] [PubMed]
- Hilton E, Rindos P, Isenberg HD. Lactobacillus GG vaginal suppositories and vaginitis. J Clin Microbiol 1995;33:1433. [Crossref] [PubMed]
- Anukam KC, Hayes K, Summers K, et al. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 may help downregulate TNF-Alpha, IL-6, IL-8, IL-10 and IL-12 (p70) in the neurogenic bladder of spinal cord injured patient with urinary tract infections: a two-case study. Adv Urol 2009;680363. [PubMed]
- Mastromarino P, Macchia S, Meggiorini L, et al. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect 2009;15:67-74. [Crossref] [PubMed]
- Parent D, Bossens M, Bayot D, et al. Therapy of bacterial vaginosis using exogenously-applied Lactobacilli acidophili and a low dose of estriol: a placebo-controlled multicentric clinical trial. Arzneimittelforschung 1996;46:68-73. [PubMed]
- Eriksson K, Carlsson B, Forsum U, et al. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta Derm Venereol 2005;85:42-6. [Crossref] [PubMed]
- Bradshaw CS, Pirotta M, De Guingand D, et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial. PLoS One 2012;7:e34540. [Crossref] [PubMed]
- Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol 2010;203:120.e1-6. [Crossref] [PubMed]
- de Vrese M, Schrezenmeir J. Probiotics and non-intestinal infectious conditions. Br J Nutr 2002;88:S59-66. [Crossref] [PubMed]
- Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect 2004;80:8-11. [Crossref] [PubMed]
- Gunston KD, Fairbrother PF. Treatment of vaginal discharge with yoghurt. S Afr Med J 1975;49:675-6. [PubMed]
- Chimura T, Funayama T, Murayama K, et al. Ecological treatment of bacterial vaginosis. Jpn J Antibiot 1995;48:432-6. [PubMed]
- Williams AB, Yu C, Tashima K, et al. Evaluation of two self-care treatments for prevention of vaginal candidiasis in women with HIV. J Assoc Nurses AIDS Care 2001;12:51-7. [Crossref] [PubMed]
- Dani C, Biadaioli R, Bertini G, et al. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002;82:103-8. [Crossref] [PubMed]
- Bezkorovainy A, Miller-Catchpole R. Ecology of Bifidobacteria. In: Biochemistry and Physiology of Bifidobacteria. Chap 2. BocaRaton, Florida, USA: CRC Press Inc., 1989:29-72.
- Mastromarino P, Brigidi P, Macchia S, et al. Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J Appl Microbiol 2002;93:884-93. [Crossref] [PubMed]
- Petricevic L, Witt A. The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. BJOG 2008;115:1369-74. [Crossref] [PubMed]
- Larsson PG, Stray-Pedersen B, Ryttig KR, et al. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health 2008;8:3. [Crossref] [PubMed]
- Pascual LM, Daniele MB, Ruiz F, et al. Lactobacillus rhamnosus L60, a potential probiotic isolated from the human vagina. J Gen Appl Microbiol 2008;54:141-8. [Crossref] [PubMed]
- Ghane M, Babaeekhou L, Ketabi SS. Antibiofilm Activity of Kefir Probiotic Lactobacilli Against Uropathogenic Escherichia coli (UPEC). Avicenna J Med Biotechnol 2020;12:221-9. [PubMed]
- Herthelius M, Gorbach SL, Möllby R, et al. Elimination of vaginal colonization with Escherichia coli by administration of indigenous flora. Infect Immun 1989;57:2447-51. [Crossref] [PubMed]
- Asahara T, Nomoto K, Watanuki M, et al. Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother 2001;45:1751-60. [Crossref] [PubMed]
- Hutchins RG, Bailey CS, Jacob ME, et al. The effect of an oral probiotic containing lactobacillus, bifidobacterium, and bacillus species on the vaginal microbiota of spayed female dogs. J Vet Intern Med 2013;27:1368-71. [Crossref] [PubMed]
- Hughes VL, Hillier SL. Microbiologic characteristics of Lactobacillus products used for colonization of the vagina. Obstet Gynecol 1990;75:244-8. [PubMed]
- Hamilton-Miller JM, Shah S, Winkler JT. Public health issues arising from microbiological and labelling quality of foods and supplements containing probiotic microorganisms. Public Health Nutr 1999;2:223-9. [Crossref] [PubMed]
- Zhong W, Millsap K, Bialkowska-Hobrzanska H, et al. Differentiation of Lactobacillus Species by Molecular Typing. Appl Environ Microbiol 1998;64:2418-23. [Crossref] [PubMed]
- Temmerman R, Scheirlinck I, Huys G, et al. Culture-independent analysis of probiotic products by denaturing gradient gel electrophoresis. Appl Environ Microbiol 2003;69:220-6. [Crossref] [PubMed]
- Strus M, Chmielarczyk A, Kochan P, et al. Studies on the effects of probiotic Lactobacillus mixture given orally on vaginal and rectal colonization and on parameters of vaginal health in women with intermediate vaginal flora. Eur J Obstet Gynecol Reprod Biol 2012;163:210-5. [Crossref] [PubMed]
Cite this article as: Das S, Ameeruddin S. Probiotics in common urological conditions: a narrative review. Longhua Chin Med 2022;5:14.

