
Cinnabar is a naturally occurring mercuric sulfide that induces cardiotoxicity in zebrafish larvae
Introduction
Cinnabar, a well-known traditional Chinese medicine (TCM) mainly composed of naturally occurring mercuric sulfide (HgS), is used for improving vision, detoxification, insomnia, epilepsy, and palpitations, and for its sedative effects, even in some pediatric medicines according to the Chinese Pharmacopeia Commission (1). It has been widely used for its therapeutic effects for over 2,000 years, and is still popular in clinical practice in Asia, the Middle East and India (2-4). The sedative and hypnotic effects of cinnabar have been reported. Pharmacological doses of cinnabar could suppress brain serotonin (5-HT) expression, reducing 5-HT synthesis and release (5). However, the efficacy and safety of cinnabar have raised persistent concerns over the centuries because of its composition; more than 96% of cinnabar is comprised of the highly toxic element, mercury (6).
Mercury is found in three forms: inorganic, organic and elemental (7). Mercury in cinnabar (HgS) is inorganic, and considerably less toxic than other species of mercury due to its low solubility and bioavailability (8). It is still controversial whether cinnabar would be transformed into toxic in the body. Liang et al. (9) hypothesized that cinnabar could be converted into MeHg (organic mercury, the most toxic form) within the gastrointestinal bacterial flora. Further study has revealed that cinnabar is not converted into more toxic mercury (MeHg), instead existing as nontoxic mercuric polysulfides in the intestine (3,10). The commonly observed clinical toxic effects of cinnabar include neurotoxicity (11,12), ototoxicity (13), hepatotoxicity (14-16), and nephrotoxicity (15,16). Recent studies show that exposure to mercury is strongly correlated with hypertension, coronary heart disease, cardiac arrhythmias, myocardial infarction, atherosclerosis, and stoke (17-21). Cardiovascular functions are affected by chronic occupational exposure to low mercury doses (22). However, few studies have reported on the cardiotoxicity induced by cinnabar. An-Gong-Niu-Huang Pill, a preparation comprised of cinnabar and realgar, is commonly prescribed to treat acute ischemic stroke and cerebral hemorrhage in China, and pericardial edema has been observed in zebrafish (16). Although cardiotoxicity of mercury and preparations with cinnabar has been reported, the cardiotoxicity of cinnabar has not been investigated experimentally.
The zebrafish (Danio rerio) genome has been fully sequenced and closely resembles the human genome, approximately 70% of human genes are orthologous to zebrafish genes while 3,176 human disease genes are found and listed in the Online Mendelian Inheritance in Man (OMIM) database at which 82% morbid genes are associated with at least one zebrafish orthologue (23). Zebrafish larvae are visible in bright conditions, which makes it possible to assess the development of different organs (24). The heart tube, with distinguished cardiac chambers, is clearly beating by 22 hours post-fertilization (hpf), and circulation begins at 24 hpf. By 36 hpf, the heart tube has looped and provides circulation to most organs such as head and trunk (25), and almost all of the organs have fully developed by 96 hpf (26). Within the past few decades, zebrafish have become one of the most important vertebrate models for studies on development, embryology, oncology, behavior, and physiology, as well as for cardiovascular research (27). Zebrafish embryos have been used for screening many types of toxic substances, specifically for cardiotoxicity, and exhibit similar physiological responses to mammals, and even humans, on exposure to antibiotics, hormones, chemical drugs and heavy metals (27-29). Cardiac functional analysis of zebrafish typically involves the heartbeat, cardiac output (CO), stroke volume (SV), fractional shortening (FS), and vascular blood flow velocity (30). Pericardial edema in zebrafish was observed in response to cardiotoxicity (29).
In order to develop a cardiotoxicity assay physiologically relevant to humans, for evaluating safety and also understanding the mechanism underlying the toxicity of cinnabar, we exposed zebrafish embryos and larvae to cinnabar, and measured the systemic effects of cinnabar on overall embryonic development, and the heart and neuronal system in in vivo experiments.
We present the following article in accordance with the MDAR reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-44/rc).
Methods
Cinnabar and zebrafish
Silybum cinnabar, which product with code number approved by National medical products administration (NMPA) is (90)32-273, was purchased from Dishui Chemical Industry Limited Company, Xiangtan, Hunan, China, and used throughout the study.
The AB wild-type zebrafish used in this study were purchased from a local pet shop, and manipulated as described in the Zebrafish Handbook (31). Transgenic fish lines Tg (cmlc2:GFP) and Tg (mnx1:GFP) ml2 were purchased from the Zebrafish Information Network (Eugene, OR, USA). Zebrafish embryos were generated by natural pairwise mating and maintained in embryo media at the Institute of Chinese Medical Sciences (ICMS), University of Macau (32).
Zebrafish maintenance
The zebrafish embryo collection, breeding, and embryonic and larval culture procedures, as well as the microscopic observations, were performed according to the standard protocols described in the Zebrafish Handbook (33). Briefly, both zebrafish strains were maintained in a controlled environment at 28.5 ℃ on a 14 h:10 h light/dark photoperiod. Fish were fed with live brine shrimp twice a day and with general tropical fish food once a day. Zebrafish embryos were maintained under standard conditions at a temperature of 28.5 ℃ in embryo medium (13.7 mM NaCl, 0.54 mM KCl, 0.025 mM Na2HPO4, 0.044 mM KH2PO4, 0.13 mM CaCl2, 0.1 mM MgSO4, and 42 µM NaHCO3; PH 7.4) (34).
Assessment of mortality rate
Wild-type zebrafish embryos at 1 day post-fertilization (dpf) were randomly placed into a 24-well plate (27 embryos per well, 81 embryos per condition) with varying cinnabar concentrations (1, 3, 10, 30, or 100 mg/mL). A group of embryos treated with embryo medium was used as a vehicle control. The incidence of lethality was recorded every 24 h from 2–7 dpf. Mortality was determined by observing the heart beats of the zebrafish (31).
Assessment of locomotion behavior
Wild-type zebrafish larvae at 3 dpf were exposed to different concentrations of cinnabar (1, 3, 10, or 30 mg/mL) for 4 days. Zebrafish larvae at 7 dpf were randomly transferred into 96-well plates (one larva per well and 12 larvae per group). Zebrafish showing signs of excessive stress upon handling (such as rapid and disorganized swimming or immobility for 2 min) were discarded. The experiments were performed in a calm, sealed area. Zebrafish larvae were allowed to habituate to the environment of the system for 30 min before tracking began. Swimming behavior was monitored by an automated video tracking system (Viewpoint; ZebraLab, LifeSciences, France). The 96-well plates and camera were housed inside a Zebrabox and the swimming pattern and total distance moved of individual zebrafish larvae were recorded in three 10-min sessions. Statistical analysis of the total distance traveled by each zebrafish larva in the different treatment groups was performed using one-way analysis of variance (ANOVA) (31).
Assessment of motor neuron phenotype
Tg (mnx1:GFP) ml2 zebrafish embryos expressing green fluorescent protein in motor neurons were used for assessment of the motor neuron phenotype. All embryos were incubated at 28.5 ℃ in embryo medium containing 0.003% PTU (to block pigmentation) from 24 hpf, and treated with different concentrations of cinnabar for 48 h. Images were taken using an Olympus DSU (Disk Scanning Unit; Olympus, Tokyo, Japan) confocal imaging system at 72 hpf.
Morphological observation
At 24 hpf, wild-type zebrafish embryos were exposed to the indicated concentrations of cinnabar for 48 h and mounted on microscope glass slides. The morphology of the larvae, including malformations in the tail and the presence of edema, was observed under a fluorescence microscope (IX71; Olympus, Japan) at 3 dpf.
Assessment of cardiac functions
Tg (cmlc2:GFP) zebrafish embryos were used for the cardiotoxicity evaluation. All embryos were cultivated at 28.5 ℃ in embryo medium containing 0.003% PTU (to block pigmentation) from 24 hpf. At this stage, the embryos were dispensed into a 96 well plate (one embryo per well) and treated with four cinnabar concentrations (1, 3, 10, or 30 mg/mL). Embryos treated with 0.5% DMSO (Sigma-Aldrich, St. Louis, MO, USA) were used as the vehicle control. Then, plates were incubated at 28.5 ℃ for 72 h.
20-s segments of video capture was recorded at room temperature using a microscope equipped using Xcellence rt software (Olympus, Japan). Sequences were captured at speeds of at least 98 frames per second, with a frame size of 256×256 pixels, and then converted into AVI movie files. The cardiac morphology, heart rate (HR), SV, CO and FS were used some mathematic formulation to do measurement (35).
HR
Heart period, defined as the interval from the beginning of one diastole to the beginning of the next, was recorded. The standard deviations for individual embryos were than averaged to obtain the mean interbeat variability for each exposure group (N=5 embryos per group). To measure the HR, the number of sequential heart contractions in 1 minute interval was counted.
CO
The CO included the heart period (defined as the interval from the beginning of one diastole to the beginning of the next) and was calculated as CO = SV × HR.
SV
End diastolic volume (EDV) and end systolic volume (ESV) in zebrafish larvae were calculated using the formula: . SV was calculated using the formula: SV = EDV – ESV.
FS
The long axis length (a) and short axis length (b) between the myocardial borders of ventricles, at diastole and systole, respectively, were measured on images from movies. The percent FS was calculated using the formula: FS = (Diastolic diameter – Systolic diameter)/(Systolic diameter) × 100%.
Statistical analyses
All statistical analyses were performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Means ± standard error of mean of means (SEM) were calculated from all individual values. To determine statistically significant difference between the treatment and control groups, one-way ANOVA was performed followed by Dunnett’s t-test (double side). P values <0.05 were considered significant.
Ethical statement
Experiments were performed under a project license (No. UMARE-030-2017) granted by the Animal Research Ethics Committee of the University of Macau, in compliance with institutional guidelines for the care and use of animals.
Results
Cinnabar induced mortality in zebrafish larvae
The mortality of zebrafish induced by various concentrations of cinnabar was measured at 1–6 dpf. Figure 1 showed that treatment with 30 mg/mL of cinnabar resulted in significant mortality at 4 dpf (P<0.05). Cinnabar at 100 mg/mL also caused significant mortality of zebrafish larvae (P<0.05), in a time frame as short as 24 h, while none of the zebrafish larvae survived at 4 dpf. Therefore exposure to cinnabar, at 30 mg/mL or higher, for 3 days showed significant acute lethal toxicity to zebrafish larvae.

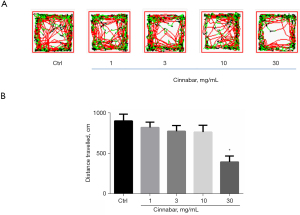
Cinnabar inhibited the locomotion of zebrafish larvae
The total distance swam in 10 minutes was analyzed as shown as Figure 2. After incubation for 4 days with various concentrations of cinnabar, the swimming distance upon treatment with 30 mg/mL cinnabar was significantly reduced (P<0.05).
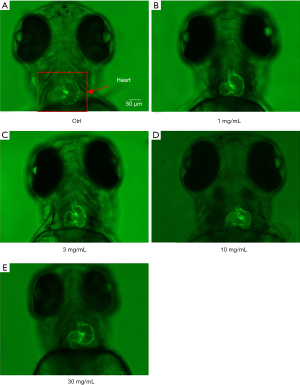
Cinnabar caused cardiac deformities in Tg (cmlc2:GFP) transgenic zebrafish larvae
Beating hearts expressing enhanced green fluorescent protein in live Tg (cmlc2:GFP) zebrafish larvae were observed by a fluorescent microscope. The effects of 1–30 mg/mL cinnabar on cardiac morphology of the larvae at 96 hpf was observed; no obvious cardiac malformations were induced in the presence of 1–30 mg/mL cinnabar, as shown as Figure 3.
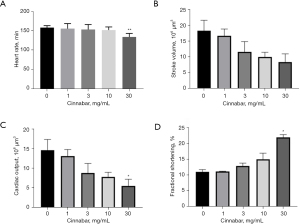
Cinnabar induced cardiac dysfunction in zebrafish larvae
To determine the toxic effects of cinnabar on cardiac functions, including the HR, CO, SV and FS of the larvae at 96 hpf were recorded. From the results shown in Figure 4, a significant decrease in HR and CO, as well as a remarkable increase in FS, were observed with 30 mg/mL cinnabar treatment (P<0.05). This indicated that exposure to cinnabar could disrupt zebrafish cardiac function in a dose-dependent manner.
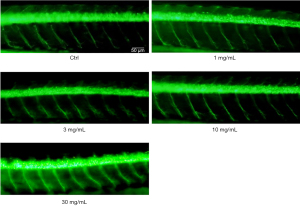
No motor neuron damage on Tg (mnx1:GFP) ml2 transgenic zebrafish larvae
The neuron system of Tg (mnx1:GFP) ml2 zebrafish expressing enhanced green fluorescent protein was observed. Motor neurons in the larvae at 72 hpf were observed under excitation by a fluorescence microscope. In Figure 5, no obvious effect on motor neurons can be seen in the larvae with the indicated concentrations of cinnabar exposure. Therefore, cinnabar exposure did not obviously affect the morphology of the motor neurons of zebrafish larvae with 48-h incubation.
Discussion
Despite the very well-documented historical use of cinnabar, the toxicity thereof is still an important concern. As mercury, a major composition of cinnabar, has been deemed highly toxic, the safety of cinnabar has been widely questioned. In the present study, we found that exposure of zebrafish embryo and larvae to cinnabar caused cardiac dysfunctions, locomotory problems and death at certain concentrations.
Cinnabar was lethal, and disrupted the normal functions of the cardiac system of zebrafish larvae, at certain dosages. A previous acute toxicity study has reported that no toxicity reactions were observed after single oral doses of 24 g/kg (2,400 ppm) of cinnabar was taken into mice, which is about 300 times the clinical equivalent dose (36). Therefore, the single dose of cinnabar is relatively safe. Although cinnabar may not cause obvious acute toxicity to adult, long-term use may increase the risk of accumulation of mercury and subsequent injury of the organs to adult and also potential risks of accidental intake to heart development in fetus and children are uncertain. It was reported that cinnabar, which contains soluble mercury (≤20 µg/g) induced pathological changes in the kidney after rats received successive doses of cinnabar (0.4 g/kg) for 4 weeks (36). It is generally recognized that drug accumulation and bioavailability are pivotal determinants of toxicological effects. Therefore, in our experiment, continuous administrations of high dosage of cinnabar (1, 3, 10, 30, or 100 mg/mL) to zebrafish embryos and larvae were tested. After exposure of zebrafish to the cinnabar from 1 to 6 dpf, we found the LC50 of cinnabar to zebrafish was between 10 mg/mL (non-lethal concentration) and 30 mg/mL (lethal concentration) (Figure 1). An effect of cinnabar on zebrafish swimming behavior was demonstrated. Exposure at 30 mg/mL cinnabar for 3 days attenuated the swimming behavior of the larvae. Also, cinnabar at 30 mg/mL caused malformation of the notochord, tail, yolk and pericardial edema, although cardiac malformations were not seen with 1–30 mg/mL cinnabar based on observations of the heart phenotype in the transgenic zebrafish larvae (Figure 3). However, exposure to 30 mg/mL of cinnabar significantly affected certain cardiac functions (Figure 4). Treatment with non-toxic concentrations (1–10 mg/mL) of cinnabar led to doses-dependent disruption of cardiac function of the larvae, but without statistical significance. However, non-lethal and lethal concentrations (1–30 mg/mL) of cinnabar did not lead to observable abnormal phenotypes of the motor neurons of the larvae (Figure 5). Whether there was a change in the electrophysiological function of the motor neurons of the larvae is uncertain and requires further examination.
The cardiotoxicity induced by cinnabar may be associated with its major component, mercury. Considerable evidence indicates that chronic exposure to low-dose inorganic mercury may also cause cardiotoxicity. The mechanism underlying cardiotoxicity on exposure to mercury may involve increased reactive oxygen species (ROS), oxidative stress and inflammation, as well as a reduction of antioxidant enzyme activity, thrombosis, mitochondrial dysfunction, depolarization, autoxidation of the inner mitochondrial membrane, and inactivation of paraoxonase (which indirectly induces hypercholesterolemia) (17,37). The mechanism underlying cinnabar-induced ototoxicity and neurotoxicity has been found to involve oxidative stress signaling, decreased Na+/K+-ATPase activities, and the increase of NO production (11,13). Based on these findings, we hypothesized that the cardiotoxicity of cinnabar may involve a similar mechanism to that of mercury.
Our results suggest that zebrafish embryos/larvae are a sensitive in vivo system to assess the chronic cardiac dysfunction caused by HgS or other components of cinnabar. The results pertaining to cardiac morphology and dysfunction parameters indicated that cinnabar may have cardiotoxic effects in mammals. It should be noted that the sulfide forms of mercury are only used in traditional oral medicines and clinical preparations; they are not but not used alone in remedies (4). Cinnabar-containing medicines are non-toxic at therapeutic doses in comparison with other forms of inorganic mercury (38). Compared with the cardiovascular toxic effects of inorganic mercury, different molecular mechanisms might underlie the pharmacological and toxicological effects of cinnabar. To date, the toxicological effect of cinnabar on the cardiovascular system have not been clearly demonstrated. These results improve our understanding of cinnabar’s toxic effects in an experimental model of zebrafish, and show the feasibility of using zebrafish embryo/larvae as an alternative in vivo assay to determine the toxicity and quality of cinnabar in products on the market.
In summary, our data demonstrated that cinnabar could induce cardiac damage and locomotory problems in zebrafish larvae. Although cinnabar did not cause significant cardiac dysfunction at the low concentrations tested herein, the potential risks of long-term exposure to low doses remain unknown. The use of cinnabar may pose a significant risk to certain populations, such as pregnant women, children, and patients with cardiac conditions.
Acknowledgments
Funding: This research was funded by The Science and Technology Development Fund, Macau SAR (File No. 0058/2019/A1 and 0016/2019/AKP), and by the University of Macau (MYRG2019-00105-ICMS and CPG2021-00011-ICMS).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Zhi-Xiu Lin, Yanfang Xian and Hongxi Xu) for the series “Pharmacology of Chinese Herbal Medicine” published in Longhua Chinese Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-44/rc
Data Sharing Statement: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-44/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-44/coif). The series “Pharmacology of Chinese Herbal Medicine” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhonghua Renmin Gongheguo wei sheng bu yao dian wei yuan hui. Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Press, 2000.
- Burger R L, Leikin J B. Cinnabar use in Prehispanic Peru and its possible health consequences. J Archaeol Sci 2018;17:730-4.
- Zhou X, Wang L, Sun X, et al. Cinnabar is not converted into methylmercury by human intestinal bacteria. J Ethnopharmacol 2011;135:110-5. [Crossref] [PubMed]
- Liu J, Wei LX, Wang Q, et al. A review of cinnabar (HgS) and/or realgar (As4S4)-containing traditional medicines. J Ethnopharmacol 2018;210:340-50. [Crossref] [PubMed]
- Wang Q, Yang X, Zhang B, et al. The anxiolytic effect of cinnabar involves changes of serotonin levels. Eur J Pharmacol 2007;565:132-7. [Crossref] [PubMed]
- Lu YT, Qi WZ, Wang S, et al. Toxicity and risk assessment of mercury exposures from cinnabar and Baizi Yangxin Pills based on pharmacokinetic and tissue distribution studies. J Ethnopharmacol 2020;250:112489. [Crossref] [PubMed]
- Syversen T, Kaur P. The toxicology of mercury and its compounds. J Trace Elem Med Biol 2012;26:215-26. [Crossref] [PubMed]
- Tinggi U, Sadler R, Ng J, et al. Bioavailability study of arsenic and mercury in traditional Chinese medicines (TCM) using an animal model after a single dose exposure. Regul Toxicol Pharmacol 2016;76:51-6. [Crossref] [PubMed]
- Liang AH, Shang MF. General situation of the study on the toxicity of Cinnabaris. Zhongguo Zhong Yao Za Zhi 2005;30:249-52. [PubMed]
- Zhou X, Zeng K, Wang Q, et al. In vitro studies on dissolved substance of cinnabar: chemical species and biological properties. J Ethnopharmacol 2010;131:196-202. [Crossref] [PubMed]
- Young YH, Chuu JJ, Liu SH, et al. Neurotoxic mechanism of cinnabar and mercuric sulfide on the vestibulo-ocular reflex system of guinea pigs. Toxicol Sci 2002;67:256-63. [Crossref] [PubMed]
- Huang CF, Liu SH, Lin-Shiau SY. Neurotoxicological effects of cinnabar (a Chinese mineral medicine, HgS) in mice. Toxicol Appl Pharmacol 2007;224:192-201. [Crossref] [PubMed]
- Huang CF, Hsu CJ, Liu SH, et al. Ototoxicity induced by cinnabar (a naturally occurring HgS) in mice through oxidative stress and down-regulated Na(+)/K(+)-ATPase activities. Neurotoxicology 2008;29:386-96. [Crossref] [PubMed]
- Lu YF, Wu Q, Liang SX, et al. Evaluation of hepatotoxicity potential of cinnabar-containing An-Gong-Niu-Huang Wan, a patent traditional Chinese medicine. Regul Toxicol Pharmacol 2011;60:206-11. [Crossref] [PubMed]
- Wei L, Liao P, Wu H, et al. Toxicological effects of cinnabar in rats by NMR-based metabolic profiling of urine and serum. Toxicol Appl Pharmacol 2008;227:417-29. [Crossref] [PubMed]
- Chen J, Liu T, Huang DP, et al. Developmental toxicity and potential teratogenicity of compound danshen tablet, angong niuhuang pill, and lidan paishi tablet in zebrafish embryos. Chin Herb Med 2017;9:74-9. [Crossref]
- Genchi G, Sinicropi MS, Carocci A, et al. Mercury exposure and heart diseases. Int J Environ Res Public Health 2017;14:74. [Crossref]
- Sogame Y, Tsukagoshi A. Development of a liquid chromatography-inductively coupled plasma mass spectrometry method for the simultaneous determination of methylmercury and inorganic mercury in human blood. J Chromatogr B Analyt Technol Biomed Life Sci 2020;1136:121855. [Crossref] [PubMed]
- Sevim Ç, Doğan E, Comakli S. Cardiovascular disease and toxic metals. Curr Opin Toxicol 2020;19:88-92. [Crossref]
- Yoshizawa K, Rimm EB, Morris JS, et al. Mercury and the risk of coronary heart disease in men. N Engl J Med 2002;347:1755-60. [Crossref] [PubMed]
- Virtanen JK, Voutilainen S, Rissanen TH, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol 2005;25:228-33. [Crossref] [PubMed]
- Furieri LB, Fioresi M, Junior RF, et al. Exposure to low mercury concentration in vivo impairs myocardial contractile function. Toxicol Appl Pharmacol 2011;255:193-9. [Crossref] [PubMed]
- Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496:498-503. [Crossref] [PubMed]
- Parichy DM, Elizondo MR, Mills MG, et al. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 2009;238:2975-3015. [Crossref] [PubMed]
- Stainier DY, Fouquet B, Chen JN, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996;123:285-92. [Crossref] [PubMed]
- Kimmel CB, Ballard WW, Kimmel SR, et al. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253-310. [Crossref] [PubMed]
- Zakaria ZZ, Benslimane FM, Nasrallah GK, et al. Using zebrafish for investigating the molecular mechanisms of drug-induced cardiotoxicity. Biomed Res Int 2018;2018:1642684. [Crossref] [PubMed]
- Yan Z, Huang X, Xie Y, et al. Macrolides induce severe cardiotoxicity and developmental toxicity in zebrafish embryos. Sci Total Environ 2019;649:1414-21. [Crossref] [PubMed]
- Zhu JJ, Xu YQ, He JH, et al. Human cardiotoxic drugs delivered by soaking and microinjection induce cardiovascular toxicity in zebrafish. J Appl Toxicol 2014;34:139-48. [Crossref] [PubMed]
- Shin JT, Pomerantsev EV, Mably JD, et al. High-resolution cardiovascular function confirms functional orthology of myocardial contractility pathways in zebrafish. Physiol Genomics 2010;42:300-9. [Crossref] [PubMed]
- Liao Q, Li S, Siu SWI, et al. Novel neurotoxic peptides from Protopalythoa variabilis virtually interact with voltage-gated sodium channel and display anti-epilepsy and neuroprotective activities in zebrafish. Arch Toxicol 2019;93:189-206. [Crossref] [PubMed]
- Liao Q, Gong G, Siu SWI, et al. A novel ShK-like toxic peptide from the transcriptome of the cnidarian palythoa caribaeorum displays neuroprotection and cardioprotection in zebrafish. Toxins (Basel) 2018;10:238. [Crossref] [PubMed]
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). 4th ed. Eugene: Univ. of Oregon Press, 2000.
- Gong G, Kam H, Tse YC, et al. Forchlorfenuron (CPPU) causes disorganization of the cytoskeleton and dysfunction of human umbilical vein endothelial cells, and abnormal vascular development in zebrafish embryos. Environ Pollut 2021;271:115791. [Crossref] [PubMed]
- Chan JY, Zhou H, Kwan YW, et al. Evaluation in zebrafish model of the toxicity of rhodamine B-conjugated crotamine, a peptide potentially useful for diagnostics and therapeutics. J Biochem Mol Toxicol 2017;31: [Crossref] [PubMed]
- Tian JZ, Liang AH, Zhu XX, et al. Advances in the safety evaluation of mineral medicines-Cinnabar and Realgar. World J Tradit Chin Med 2019;5:164-72. [Crossref]
- Räsänen L, Mutanen M. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 1995;92:2355-6. [PubMed]
- Liu J, Shi JZ, Yu LM, et al. Mercury in traditional medicines: is cinnabar toxicologically similar to common mercurials? Exp Biol Med (Maywood) 2008;233:810-7. [Crossref] [PubMed]
Cite this article as: Un EMW, Chan ESY, Lam KH, Li L, Lee SMY. Cinnabar is a naturally occurring mercuric sulfide that induces cardiotoxicity in zebrafish larvae. Longhua Chin Med 2022;5:2.

