
A narrative review on pharmacological significance of Eurycoma longifolia jack roots
Introduction to Eurycoma longifolia
Eurycoma longifolia Jack is a slender tree from the family Simaroubaceae. This plant is widely distributed in the tropical forest of Southeast Asia. It is popularly known as the king of herbs and locally recognized as ‘Tongkat Ali’ which literally means “Ali’s walking stick”. The plant gets the name because it has long-twisted roots (1). Sometimes, the plant is called as Malaysian Ginseng, and the other names include Payung Ali, Penawar Pahit, Setunjang Bumi, Bedara Pahit, Tongkat Baginda, Pokok Syurga, Tongkat Ali Hitam, Pokok Jelas and Jelaih in Malaysia (2). In South East Asian, the plant is commonly known as ‘Pasakbumi’ in Indonesia, ‘Cay ba binh’ in Vietnam and ‘Ian-don’ in Thailand.
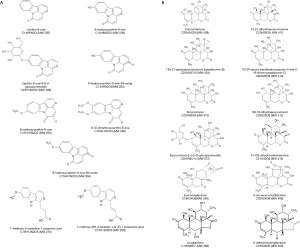
The roots of E. longifolia has been well documented for its medicinal values such as antimalarial, anti-inflammatory and aphrodisiac effects. The ethnomedicinal knowledge of E. longifolia has been continuously and widely applied by indigenous people till to date. In particular, the plant roots are believed to have higher therapeutic effects than other parts of the plant. Recent scientific studies have also proven the remarkable pharmacological properties of the plant, mostly the plant roots. This can be seen from the previous reported findings which have been gathered and systematically compiled in Table 1. The chemical structures of the reported compounds from E. longifolia extracts are presented in Figure 1. The data reveals that scientific studies on the pharmacological properties of the plant have been extensively carried out since 1980s. The promising results have also boosted the application of the plant extracts and the acceptance of public using E. longifolia in health promoting interest. The technical evidences explained that the ethnopharmacological applications were mainly contributed by phytochemicals in the plant roots. The major classes of phytochemicals, particularly quassinoids, canthin-6-one alkaloids, β-carboline alkaloids, squalene derivatives and biphenylneolignans have been reported to possess the pharmacological significance (42). Most of the reported compounds in E. longifolia belong to the group of quassinoids or degraded triterpenes. One of the commonly known quassinoids is eurycomanone which is also a C 20-type quassinoids and the marker compound of the plant.
Table 1
| Part of plant | Pharmacological property | Cell line/strain/activity | Compound | References |
|---|---|---|---|---|
| Root | Anticancer | Human cancer cell types (breast, colon, fibrosarcoma, lung, melanoma, KB) | 9-methoxycanthin-6-one | (3) |
| 9-methoxycanthin-6-one-N-oxide | ||||
| 9-hydroxycanthin-6-one | ||||
| 9-hydroxycanthin-6-one-N-oxide | ||||
| Eurycomanone | (3-5) | |||
| Human breast cancer cell line (MCF-7), human gastric cancer cell line (MGC-803) | 5-iso-eurycomadilactone | (6) | ||
| Eurycomanone | ||||
| Human breast cancer cell line (MCF-7), human lung cancer cell line (A549) & human cervical cancer cell (HeLa) | Eurycomalactone | (7) | ||
| Longilactone | ||||
| 14,15β-dihydroxyklaineanone | ||||
| Human breast cancer cell lines (T47D & MCF-7), human cervical cancer cell (HeLa) and human colon carcinoma cell line (WIDR) | Eurycomanone | (8) | ||
| Human breast cancer cell line (MCF-7) & human lung cancer cell line (A-549) | Eurycomalactone | (9) | ||
| 6-dehydroxylongilactone | (10) | |||
| Longilactone | (11) | |||
| Canthin-6-one | (12) | |||
| 9-methoxycanthin-6-one | (12) | |||
| 14,15β-dihydroxyklaineanone | (11) | |||
| Canthin-6-one 9-O-β-glucopyranoside | (12) | |||
| 13β,21-epoxyeurycomanone (pasakbumin B) | (4) | |||
| 11β,20-epoxy-pentahydroxypicras-3-ene-2,16-dione (pasakbumin C) | (4) | |||
| Human breast cancer cell lines |
Eurycomanone | (13) | ||
| Human breast cancer cell line (MCF-7) | Longilactone | (14) | ||
| Eurycomanone | (15,16) | |||
| Human breast cancer cell line | Extract | (17) | ||
| Human leukemia cell lines (HL-60 & Jurkat) | Eurycomanone | (18) | ||
| Human leukemic cell line (K-562) | Extract | (19) | ||
| Human lung cancer cell line (A549) | Eurycomanone | (20) | ||
| Human liver cancer cell (HepG2) | Eurycomanone | (21) | ||
| Stem | Human fibrosarcoma cell line (HT-1080) | Eurycomalactone | (11) | |
| 13,21-dihydroeurycomanone | (22) | |||
| 14,15-dihydroxyklaineanone | ||||
| Root | Antimalarial | Plasmodium falciparum | Eurycomanone | (3,23) |
| Eurycomanol | (23) | |||
| 13,18β-dihydroeurycomanol | ||||
| Eurycomanol-2-O-β-D-glucopyranoside | ||||
| 13β,21-epoxyeurycomanone (pasakbumin B) | (5) | |||
| 7-methoxy-carboline-1-propionic acid (ß-carboline alkaloids) | (3) | |||
| Plasmodium falciparum (chloroquine-resistant Gombak A and chloroquine-sensitive D10 strain) | Eurycomanone | (24) | ||
| 13,21-dihydroeurycomanone | ||||
| Toxoplasmicidal evaluation against Vero and HS27 cell lines | Unknown | (25) | ||
| Root | Anti-leukemic/anti-inflammation | NF-κB inhibitor (IκBα phosphorylation and upstream mitogen activated protein kinase signaling) | Eurycomanone | (26) |
| NF-κB inhibitor | Eurycomalactone | (27) | ||
| 13,21-dihydroeurycomanone | ||||
| 14,15β-dihydroklaieanone | ||||
| Suppression of pro-inflammatory |
9,10-dimethoxycanthin-6-one | (28) | ||
| 7-methoxy-(9H-β-carbolin-1-il)-(E)-1-propenoic acid (β-carboline alkaloid) | (29) | |||
| Edema thickness (in vivo) | Extract | (30) | ||
| Root | Antihyperglycaemic | Lower blood glucose (in vivo) | Extract | (31) |
| Lower blood glucose and lipid-lowering effects (in vivo) | Extract | (32) | ||
| Inhibitory activity of advanced glycation end-products (AGEs) formation (excess glycation and thus is believed to cause diabetic complications) | Extract | (33) | ||
| Lower blood glucose (in vivo) | Extract | (34) | ||
| Lower blood glucose (in vivo) | Extract | (35) | ||
| Reduce alpha-2-HSglycoprotein(AHS) in serum | Extract | (36) | ||
| Unknown | Increase insulin sensitivity through the enhancement of glucose uptake and reduce lipid accumulation in adipose cells | Extract | (37) | |
| Unknown | Anti-hypertriglyceridemic | Decrease high-density lipoprotein (in vivo) | Extract | (30) |
| Root | Anti-osteoporosis | High dose (>90 mg/kg) increase bone microarchitecture (in vivo) | Extract | (38) |
| Root | Aphrodisiac | Sexual enhancement | Extract | (36) |
| Lower blood pressure | Extract | (39) | ||
| Root | Antiadipogenic (obesity) | Inhibit adipogenesis | Extract | (40) |
| Increase lipolysis | Eurycomanone | (41) | ||
| 13β,21-epoxyeurycomanone (pasakbumin B) | ||||
| Unknown | Analgesic | Reduce in writhing (in vivo) | Extract | (30) |
Therefore, the objective of this narrative review is to draw conclusive information from all published data on the pharmacological significance of E. longifolia. Previous review articles on E. longifolia have been published from 2010 onwards, they mostly report on individual pharmacological properties and clinical studies. The present review is mainly focused on a broad range of reported pharmacological significance in relation with phytochemicals. The well-organized information is very important to rationale future studies, and leading to the drug development based on the potentials of phytochemicals in the plant extract. We present the following article in accordance with the Narrative Review checking checklist (available at https://dx.doi.org/10.21037/lcm-21-32).
Pharmacological properties of Eurycoma longifolia
Aphrodisiac effects of Eurycoma longifolia
E. longifolia has been traditionally gained the attention of local folks because of its promising efficacy in sexual enhancement. Research findings have also proved its potency to treat male sexual disorder and improve overall sexual wellbeing in men. Many experimental works on the aphrodisiac effect of E. longifolia extract were performed in rodents, mostly in sexually normal male rats (43), in sexually naïve male rats and mice (44,45), in middle-aged rats and mice (46-48) and sexually sluggish old rats (49). The observation proven the extract of E. longifolia could improve sexual behavior significantly. Sexual performance test showed that E. longifolia could improve the initiation of sexual actions like mounting, intromission and ejaculation in inexperienced castrated male rats upon oral administration of E. longifolia extract in a dose-dependent manner. E. longifolia was also able to stimulate the development of ventral prostate and seminal vesicles in castrated male rats (50). In addition, 12 weeks successive oral administration of different fractions of E. longifolia increased the weight of bilateral laevator ani muscle in intact male rats, following the protein anabolic action of androgens which explained the pro-androgenic effect of E. longifolia (51).
A study on sexually sluggish male rats showed that the decease of hesitation time to cross electric grid after administration of E. longifolia fractions (water, methanol, butanol and chloroform) at a dose of 0.5 g/kg (52). In addition, the administration of E. longifolia fractions caused a transient increase in the percentage (up to more than 50%) of male rats responding to the right choice using the electrical copulation cage (52). Somehow, the researchers were unable to conclude which fraction was more efficient in contributing the aphrodisiac property. Therefore, no conclusive findings on the contributor group of phytochemicals in enhancing aphrodisiac property. In other studies, reported by the same research group that an enhancement of sexual motivation was observed from the increment of adult middle-aged male mice and retired breeders responding to the right choice after the consumption of various extracts (47) and fractions (48) of E. longifolia. E. longifolia extracts also exhibited the sex drive stimulus such as yawning and stretching by 50% and 16.7%, respectively in sexually sluggish old male rats (49).
Previous studies reported significant increment in serum testosterone level of rats treated with the plant root powder for 6 days, and subsequently resulted in the improvement of copulatory activity (53). The acute administration of the highest dose (1,000 mg/kg) increased the percentage of mounting and ejaculating rats, particularly in sexually sluggish rats (75% and 62.5%, respectively) when compared to the controls (25%). Moreover, the subacute and subchronic treatments increased the percentage of mounting rats from 0% (controls) to 25% and 37.5%, respectively, and the percentage of ejaculating rats from 0% (controls) to 25% in both treated groups of impotent rats. On the other hand, better results related to ejaculation latency and post-ejaculatory interval were obtained after the subacute administration (6 days) in sexually sluggish rats in comparison with the acute and the subchronic (12 days) administrations. However, a single administration of 500 or 1,000 mg/kg of the plant root powder reduced ejaculation latency. This could be due to the excessive doses of administration to the rats. A dose range of 100–200 mg/kg for in vivo studies of extracts or 100–200 µg/mL should be assumed as a meaningful upper limit for pharmacological studies (54).
Clinical trials for male infertility
Apart from animal studies, there were also several human clinical trials conducted to investigate the therapeutic feasibility of E. longifolia in managing male sexual disorders including erectile dysfunction, male infertility, low libido, and downregulated testosterone levels. Neurological, vascular, and hormonal disorders are major factors that contribute to the sexual dysfunction in male along with bad diet and lifestyle (55). Erectile dysfunction, a frequent and persistent inability of male to attain and/or to retain penile erection during the course of performing sexual activity is one of the primary causes of dissatisfaction in sexual performance (56,57). Physta, a proprietary freeze-dried water extract of E. longifolia root notably improved scores for the sexual intercourse attempt diary, erection hardness scale, sexual health inventory of men, and aging male symptom scale (P<0.05 for all) in a 12-week randomized, double-blind, and placebo-controlled parallel-group study consisted of men aged from 40 to 65 years old. Besides, there was no clinically significant changes in the vital organs of body such as liver and kidney by laboratory evaluation (58).
Erectile dysfunction is remarkably influenced by age and prevalence among men between 70ties and 80ties of age (59). The decreased levels of testosterone and inadequate blood supply flowing to penis induce erectile dysfunction. cGMP-specific phosphodiesterase type 5 inhibitors such as sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra) are the commonly prescribed medications to treat erectile dysfunction. These drugs improve penile erection by regulating blood flow to the penis (58). E. longifolia is a potent alternative medicine for erectile dysfunction without any of side effects associated with medicines such as visual disturbances, flushing, muscle pain, and back pain (60).
E. longifolia was found to facilitate conversion of pregnenolone to progesterone, cortisol, 5-dehydroepiandrosterone, and testosterone in rabbit corpus cavernosum tissues (58). Eurypeptides, the bioactive complex polypeptides with a molecular weight of 4.3 kDa exerted its effects on the biosynthesis of various androgens. CYP17 (17α-hydroxylase/17,20 lyase) is activated by eurypeptides to enhance the metabolism of pregnenolone and 17-OH-pregnenolone to yield dehydroepiandrosterone, 4-androstenedione and testosterone (61). CYP17 is a P450 enzyme that catalyzes the last step of androgen biosynthesis in both testis and adrenals (62). Eurypeptides increase the release rate of free form of testosterone from its binding proteins, sex hormone-binding-globulin. The testosterone balancing effects of this water soluble glycoprotein extract showed to deliver anti-aging and anti-stress benefits (63). A study was conducted to investigate the efficacy of a patented standardized water-soluble extract of E. longifolia in the treatment of 76 hypogonadic men suffering from late onset hypogonadism. The results showed a significant improvement (P<0.0001) in the serum testosterone concentration from 5.66±1.52 to 8.31±2.47 nM, which denoted an average increase of 46.8% (61).
Several human clinical studies also depicted the effects of E. longifolia in managing male infertility. Men with idiopathic male infertility characterized by low sperm concentration with or without low motility and with normal morphology were treated with 200 mg of patented standardized water-soluble extract of E. longifolia root daily for 9 months (64). Subsequent semen analysis was conducted for every 3 months based on the World Health Organization guidelines (65). The results showed the extract significantly improved (P<0.05) sperm qualities of patients such as improvement in semen volume, sperm concentration, sperm motility and percentage of morphologically normal sperm, allowing for 11 (14.7%) spontaneous pregnancies. In addition, a randomized, double-blind, placebo controlled, parallel group study investigated the aphrodisiac clinical evidence of E. longifolia extract in accordance with the Good Clinical Practice (ICH-6) guidelines and Declaration of Helsinki in 109 men between the ages of 30 to 55 years. The results showed higher scores in the overall erectile-function-domain (P<0.001), the sexual libido (14% by week 12), seminal fluid analysis-with sperm motility at 44.4%, and semen-volume at 18.2% following 12-week treatment with 300 mg of freeze-dried water extract of E. longifolia daily. The results of the study also demonstrated the positive effect of E. longifolia on male fertility with considerable positive effects on seminal parameters such as volume and concentration of sperms in ejaculate and the proportion of sperms having normal motility (59).
Antiproliferation of cancer cells
The potential of Eurycoma longifolia as anti-cancer agent has been proven in a number of studies. Previous studies reported the anticancer properties of E. longifolia on different kinds of cancerous cell lines and solid tumors including lung, breast and cervical cancers. Extracts from the leaves, stems and roots, as well as isolated bioactive compounds have been thoroughly tested for the anti-tumor effect. 14,15β-dihydroxyklaineanone isolated from the plant leaves was found to be the most active compound to promote anti-tumor property by inhibiting tumor promoter-induced Epstein-Barr virus activation with the IC50 value of 5 µM (66). The value was comparatively higher than that of quercetin (IC50=23 µM) and β-carotene (IC50=30 µM) which are two commonly known as natural anti-cancer agents (66). Among the 65 compounds, Kuo et al. (5) revealed that some compounds (eurycomalactone, 6-dehydroxylongilactone, 9-methoxy-canthin-6-one, canthin-6-one, longilactone, 14β, 15β-dihydroxyklaineanone, pasakbumin C and canthin-6-one 9-O-β-glucopyranoside) demonstrated strong cytotoxicity toward human lung cancer (A-549) cell lines, while some compounds (eurycomalactone, 6-dehydroxylongilactone, 9-methoxy-canthin-6-one, 14,15β-dihydroxyklaineanone, eurycomanone, pasakbumin B and pasakbumin C) exhibited strong cytotoxicity toward human breast cancer (MCF-7) cell lines. Moreover, Chuen and Pihie (67) showed that the semi purified eurycomanone also possessed toxicity towards breast cancer cells, but was relatively non-toxic to non-cancerous breast cells (MCF10A). These are interesting findings to prove the feasibility of phytochemicals in E. longifolia extracts as potential leads in drug development.
Delineating cellular mechanism against cancer cells
Several studies were also attempted to investigate the cellular mode of action attributed to phytochemicals in anti-tumor promoting properties. The cytotoxic activities of E. longifolia prominently followed the pathway of programmed cell death of apoptosis (16). The methanolic fraction from the plant roots selectively exhibited higher cytotoxic activity towards human breast cancer cell line, MCF-7 with the IC50 value of 15.23±0.66 µg/mL without affecting normal breast cells (MCF-10A). That fraction demonstrated the growth inhibition of MCF-7 cells through apoptotic cell death by maintaining the pro-apoptotic Bax protein levels and decreasing the anti-apoptotic Bcl-2 protein expression (16). A similar mode of action was also noticed by Mahfudh and Pihie (68) who reported that eurycomanone showed anticancer activity against cervical carcinoma HeLa cells. Eurycomanone triggered apoptosis by promoting the up-regulation of p53 tumor suppressor protein which was followed by the increasing of pro-apoptotic Bax protein and the decreasing of anti-apoptotic Bcl-2 (21). They also discovered that eurycomanone considerably reduced the viability and proliferation of cancer cells such as CaOv-3, HeLa, HepG2, HM3KO and MCF-7 in a concentration dependent manner. Park et al. (7) concluded that eurycomalactone, eurycomanone, longilactone and 14, 15β-dihydroxyklaineanone showed to be cytotoxic to MCF-7 among the 15 compounds investigated in their studies. In addition, the methanolic fraction also induced caspase activation to mediate apoptosis in MCF-7 cells. The role of that fraction was to induce the cell death of MCF-7 cell death, and followed by the cleavage and activation of caspase-7 which led to the specific proteolytic cleavage of poly(ADP-ribose) polymerase-1 (69). As reported by Muhamad et al. (14), longilactone in E. longifolia extract induced apoptosis in MCF-7 via an extrinsic pathway. Meng et al. (6) concluded that quassinoids showed the best inhibitory activity on MCF-7 cancer cells among the cancer cell lines such as HT-29, MCF-7, LOVO, BGC-823, MGC-803, HepG2, HeLa, and A549.
Al-Salahi et al. (19) conducted a study to assess in vitro and in vivo anti-proliferative and apoptotic potentials of E. longifolia on K-562 leukemic cell line. Their findings indicated that standardized methanolic extract (TAF-273) and eurycomanone could induce cellular responses in K-562 cells. The anti-proliferative effect of TAF-273 on K-562 cells could be attributed primarily to the induction of cell arrest at the G1 and S phase which mostly targeted at cell division and DNA synthesis. Apart from cell cycle arrest, TAF273 also inhibited cell proliferation by preventing the formation of blood vessels. Cancerous cells that undergo apoptosis are generally characterized by condensed and fragmented nuclei which could be observed after Hoechst staining (16). Most cancerous cells showed the apoptotic features after treated with E. longifolia extract (19,68).
A standardized extract containing 40% of total quassinoids (SQ40) from E. longifolia inhibited LNCaP human prostate cancer cell growth at the IC50 value of 5.97 µg/ml by suppressing the expression of regulatory proteins involved in G1-to-S transition of cell cycle such as CDK4, CDK2 and Cyclin D1 (70). SQ40-treated LNCaP cells also demonstrated the up-regulation of expression level of the Cyclin inhibitor protein p21Waf1/Cip1. SQ40 also suppressed the growth of LNCaP cells through the inhibition of androgen receptor activities. The suppression of androgen receptor translocation from cytoplasm into nucleus could prevent the transcription of androgen-responsive genes, thus significantly reducing the secretion of prostate-specific antigen.
It was found that eurycomanone promoted cell apoptosis through the inhibition of NF-κB signaling pathway by inhibiting phosphorylation of IκBα and upstream mitogen activated protein kinase signaling in Jurkat and K562 human leukemia cell models in a low micromolar range (26). The recent findings reported by Tung et al. (18) also proved the selectively potential antiproliferation of eurycomanone on leukemia cell lines (HL-60 and Jurkat) and low toxic effects on normal skin fibroblast cell line (NB1RGB). Eurycomanol was unable to have such inhibitory action against leukemia cells, probably due to the lack of α,β-unsaturated ketone in the molecular structure of compound (26). Eurycomalactone, 13,21-dehydroeurycomanone, and 14,15β-dihydroklaieanone are also the quassinoids compounds that exhibited strong inhibition for NF-κB with IC50 values less than 1 µM (0.5, 0.7, and 1.0 µM, respectively) (27).
Antimalarial properties of Eurycoma longifolia
Plasmodium falciparum is the most deadly and complicated form of parasite species which causes malaria and resistant against chloroquine treatment (23). Hence, it is important to find alternatives of drugs against chloroquine-resistant P. falciparum. E. longifolia or its bioactive compound is likely to be a potential lead. Its decoction has traditionally used to treat malarial fevers. The traditional usage of the plant extract also shows a promising anti-malarial effect against P. falciparum. Scientifically, studies have intensively proven the anti-malarial activities of the plant extract and its bioactive compounds against chloroquine-resistant malaria.
The evaluation was conducted on the ethanolic extract of E. longifolia dried root from Thailand. The SYBR green I-based assay indicated a great antimalarial activity with the IC50 of 2.16 and 1.79 µg/mL against P. falciparum strain 3D7 (chloroquine sensitive) and K1 (chloroquine resistant), respectively (71). Among different extracts of E. longifolia from Cambodia, the dichloromethane extract of the plant bark was proved to be the most active extract against multidrug-resistant P. falciparum strain (W2) with the IC50 value of 1.7 µg/mL (72). Possibly, eurycomanone and pasakbumin B from the plant attributed to the potent antimalarial activity against two resistant P. falciparum clones, W2 (IC50=14.912 and 22.658 ng/mL, respectively) and D6 (IC50=26.094 and 34.001 ng/mL, respectively). While the IC50 values of Chloroquine for W2 and D6 were 75.270 and 2.722 ng/mL, respectively (5,72). However, the inhibitory activity of the standardized E. longifolia extract containing three major quassinoids, namely eurycomanone, 13,21-dihydroeurycomanone, and 13α(21)-epoxyeurycomanone against P. falciparum schizont maturation was higher than that for individual quassinoids. The observation explains the synergistic effect between the quassinoids, or possibly the presence of other unidentified compounds in the extract (73).
There was also a study to test the in vitro anti-malarial activity of quassinoids rich E. longifolia extract against six chloroquine-resistant P. falciparum isolated from Malaysia (23). The quassinoids rich extract inhibited the growth of parasites in a dose-dependent manner. The result was comparable to that of chloroquine treatment. A complete inhibition was also noticed at the concentration range of 1.25–5.00 µg/mL extract after 3 days of post-treatment (23). Eurycomanone was one of the quassinoids which exhibited high antiplasmodial activity compared to the chloroquine treatment against chloroquine-resistant (3,5,24) or multidrug-resistant (9) P. falciparum. The findings revealed that eurycomanone was found to be 8.66 times more potent than chloroquine diphosphate against the isolate. The presence of an α,β-unsaturated ketone at C-2 in ring A of eurycomanone was reported to confer the strong antiplasmodial activity of eurycomanone (24). The other eurycomanone derivatives such as 13,21-dihydroeurycomanone and 13,21-epoxyeurycomanone were also more potent than chloroquine diphosphate against the Gombak A isolate of P. falciparum, whereas eurycomanol was found to be less potent compound in antimalaria.
The antiplasmodial potential of E. longifolia could be explained by the inhibitory action of glutathione (GSH) synthesis (74). Plasmodium grows inside oxygen-rich erythrocytes by consuming haemoglobin and releasing reactive oxygen species. This leads to the formation of superoxides anion and haem. GSH conjugation in haem polymerization process would detoxify superoxides anion and haem, and thus assisting the growth of parasites. High level of GSH synthesis would protect the drug resistant Plasmodium in which chloroquine acts as a competitive inhibitor of the GSH conjugation. The study demonstrated that the methanol extracts of E. longifolia (TA164) decreased the GSH content of both infected and healthy erythrocytes (74). The findings indicated that TA164 was able to completely inhibit the growth of P. falciparum at concentration 16 µg/mL. The inhibitory capability of TA164 was significantly higher than the GSH synthesis inhibitor, D,L-buthionine-(S,R) sulphoximine which only exhibited 95% inhibition at high concentration, 64 µg/mL.
In vivo antimalarial activity was conducted using a standardized E. longifolia root extract enriched with four major bioactive quassinoids such as eurycomanone (18.5%), 13α(21)-Epoxyeurycomanone (4.0%), 13α,21-dihydroeurycomanone (0.7%) and eurycomanol (9.5%) (75). 13α(21)-Epoxyeurycomanone and eurycomanone showed a gradual increase in plasma concentration upon oral administration of the extract at 200 mg/kg to Sprague-Dawley rats. Despite the concentration of 13α(21)-Epoxyeurycomanone was 4.6-fold lower than eurycomanone, 13α(21)-Epoxyeurycomanone possessed higher absolute bioavailability, 24.7% than that of eurycomanone which was only 2.6%. In contrast to the chemical degradation of 13α,21-dihydroeurycomanone and eurycomanol, 13α(21)-Epoxyeurycomanone and eurycomanone displayed strong pH stability towards acidic environment in gastrointestinal tract. However, eurycomanone could impart poor membrane permeability of absorption due to its highly polar nature. Meanwhile, the 13α(21)-epoxy analogue of 13α(21)-Epoxyeurycomanone possesses high lipid solubility (hydrophobicity) for absorption in gastrointestinal tract. In spite of lower bioavailability, eurycomanone showed a 1.9-fold higher antimalarial activity than 13α(21)-Epoxyeurycomanone. Therefore, the antimalarial activity was solely attributed to the synergistic effect between 13α(21)-Epoxyeurycomanone and eurycomanone.
Antiosteoporotic potentials of Eurycoma longifolia
E. longifolia has been reported for its potential as an alternative antiosteoporotic agent in the treatment of male osteoporosis caused by androgen deficiency called hypogonadism (76). In hypogonadism, the disruption of one or more levels of hypothalamic-pituitary-testicular axis caused failure to the testis to produce physiological levels of androgen, especially testosterone (62). Most bone cells have androgen receptors which play a vital role in bone formation. Androgens may protect men against osteoporosis by maintaining cancellous bone mass and expansion of cortical bone. Interestingly, E. longifolia may increase the testosterone levels and prevent bone calcium loss to preserve the bone calcium content. Testosterone was able to improve the trabecular bone volume of orchidectomised rats (62). Based on the findings reported by previous researchers, E. longifolia can possibly be used as an alternative treatment for testosterone replacement therapy to prevent and treat male osteoporosis induced by androgen deficiency without causing any side effects. Proandrogenic effects of E. longifolia could enhance testosterone level to stimulate osteoblast proliferation and osteoclast apoptosis. This maintains bone remodelling activity, and thus reducing bone loss (76).
An in vitro study using MC3T3-E1 cells as osteoblastic model was conducted to investigate the molecular mechanism of E. longifolia for the prevention and treatment of male osteoporosis (77). The results found that a dose of 25 mg/mL E. longifolia extract could stimulate bone formation through significant up-regulating the expression of various mitogenic proteins such as bone morphogenic protein-2, runt-related transcription factor-, osteocalcin, type I collagen, transforming growth factor-β1, androgen receptor, alkaline phosphatase activity and down-regulating the expression of osteopontin, a negative regulator of bone formation.
Another in vitro study conducted by Thu et al. (78) in the following year. They reported the use of RAW 264.7 cells as an osteoclastic model to investigate the effect of the plant extract on the translational mechanism of osteoclastogenesis. Cell cultures treated with standardized aqueous root extract showed a significant down-regulation of RANKL-induced tartrate-resistant acid phosphatase activity and expression of osteoclast-related protein biomarkers such as matrix metallopeptidase-9, cathepsin-K, tartrate-resistant acid phosphatase, nuclear factor of activated T-cells cytoplasmic 1. Overall, E. longifolia suppressed the generation of superoxide free radicals by enhancing superoxide dismutase activity and inhibiting the function of osteoclasts. Osteoclasts play a vital role in bone loss.
Antidiabetic effects of Eurycoma longifolia
Diabetes mellitus (DM) is a metabolic disorder of glucose metabolism which is the major chronic disease in many countries in South East Asia. DM can be categorized into two types; Type I DM is known as insulin-dependent DM which occurs due to the destruction of insulin-secreting beta-cells in the pancreatic islets, and thus leading to insufficient insulin production in the body, and Type II DM is known as non-insulin-dependent DM in which muscles, liver, and adipose tissues are insulin resistant and insensitive to insulin (32).
It is worthy to note that E. longifolia could exhibit tremendous antidiabetic potentials. Hendra et al. (30) reported that E. longifolia root extract could reduce triglyceride level compared with the control group of rats treated with diet with high glucose and fructose components. Often, high triglyceride level is associated with prediabetes or type II DM. The plant was proven to be capable of modulating glucose and lipid metabolism by increasing glucose uptake and suppressing lipid production in 3T3-L1 adipocytes simultaneously (37). Therefore, E. longifolia extract, particularly quassinoids enriched fraction may prevent the onset of obesity-associated T2DM (40). In addition to reduce intracellular lipid droplets and triglycerides, that fraction could suppress body weight gain, decrease epidydimal and perirenal fat pad mass and size. Although fed with high-fat diet, C57BL/6J mice did not show elevated total cholesterol and triglyceride levels, and fat accumulation liver.
Antihyperglycemic potentials
The antidiabetic effect of E. longifolia roots was found to be more effective using polar fraction obtained from the solvent mixture of ethyl acetate-ethanol-water in a ratio of 80:30:1. The effectiveness was determined based on the sample capacity to reduce blood glucose level in streptozotocin induced diabetic rats at the fifty-effective dose (ED50) of 1.71 mg/dL. The other sample with lesser polarity that obtained from the same solvent mixture with different ratios, 100:30:1 was recorded to have the ED50 of 67.48 mg/dL. The observation suggested that phytochemicals and their concentration might contribute to the difference of antidiabetic potentials (34).
An in vivo study on the efficacy of powdered E. longifolia roots to exhibit antihyperglycemic effect was conducted by Tsai et al. (32) recently. They reported the significant reduction from 37.7% to 64% of fasting blood glucose after one to eight weeks of treatment (100 mg/kg powdered extract) in the db/db mice (32). E. longifolia root extract could improve islet performance by increasing β-cell number, and pancreatic and duodenal homeobox 1 expression. There was no hypoglycemic response was found in the normoglycemic C57BL/6J mice, even treated with high dose, 200 mg/kg extract. The function of E. longifolia extract in antidiabetic property can also be rationalized by the reduction of alpha-2-HS glycoprotein expression in the serum protein of treated rats. Chen et al. (36) reported the decreased expression of alpha-2-HS glycoprotein in rats after treated with standardized E. longifolia extract at a dose of 50 mg/kg for consecutive 28 days.
However, it is noted that the antidiabetic property of E. longifolia was only displayed at high dose of administration. For instance, the high dosage, 150 mg/kg body weight of E. longifolia aqueous extract was able to significantly reduce the blood glucose level for about 46.8% in hyperglycaemic rats (31). In another study performed by Fransisca et al. (35), who reported that the methanol extract of E. longifolia roots demonstrated blood glucose-lowering effect at the doses of 210 and 420 mg/kg body weight.
Toxicity of Eurycoma longifolia
Generally, herbal medicines comparatively exhibit fewer side effects than allopathic medicines. Despite the various pharmacological benefits, the safety and toxicity of E. longifolia are of great importance to avoid any adverse effects upon consumption. Previously, a subacute and subchronic administration of E. longifolia extracts (0, 0.6, 1.2, and 2 g/kg body weight/day) for 4 and 13 weeks produced neither mutagenic nor clastogenic effects in rats. The acute oral of 50% lethal dose (LD50) was more than 6 g/kg body weight (79). Moreover, phytochemicals in the E. longifolia water extract did not also exhibit any adverse effects related to body weight, hematology, serum biochemistry, urinalysis, macropathology, or histopathology. However, the treatment significantly reduced prothrombin time, partial thromboplastin time, blood urea nitrogen, creatinine, aspartate aminotransferase, creatine phosphate kinase, lactate dehydrogenase, and cholesterol levels in male rats (P<0.05) which regarded as beneficiary effects.
In contrast, another acute toxicity study showed that the n-butanol extract of the plant was found to be the most toxic with a LD50 of 1.36 g/kg (80). The strong toxicity was attributable to the presence of higher concentration of eurycomanone. The compound was found to be the most toxic compound among other chemical constituents. It possessed an oral LD50 of 0.05 g/kg. A structure activity relationship showed the presence of unsaturated double bond at C-13 and C-21, an α,β -unsaturated ketone at C-2 in ring A, and the oxymethylene bridge linking C-11 to C-8 are contribute to the strong toxicity of eurycomanone. On the other hand, the water fractions of E. longifolia were considered to be safe as it demonstrated significantly less toxicity with the LD50 value of 5.27 g/kg than the extracts prepared by organic solvents. Even though it was previously reported to be toxic compound, recent data revealed that 0.137 µg/g (35% LD50) was considered to be safe for consumption based on the toxicological evaluation using catfishes (81).
Conclusions
The pharmacological importance of E. longifolia is mainly contributed by its phytochemicals, particularly quassinoids which are the major compounds in the plant roots. However, the significance of the biological activities is strongly depended upon types of quassinoids and their derivatives with different functional groups, as well as the synergistic effects in the plant extracts or fractions. Mostly, eurycomanone was reported to be the dominant contributor of pharmacological properties, particularly anticancer, antimalarial and antiinflammation of E. longifolia. Detail comparison is difficult to achieve conclusive information because many studies did not report the chemical characteristics of plant extract. Therefore, the plant root extract shall be well characterized for its phytochemical profile in order to have an insightful understanding of this traditional herb and its pharmacological relevance. Plant extract in any pharmacological studies must at least obtain from standardized extraction conditions to ensure quality consistency and data validity. The utmost importance is authenticating plant species for its botanical identity prior to any experimental works.
Acknowledgments
Funding: This work was supported by Universiti Teknologi Malaysia [UTMHR-08G84].
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Longhua Chinese Medicine for the series “Bioactive compounds from natural products with antidiabetic potentials”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/lcm-21-32
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/lcm-21-32). The series “Bioactive compounds from natural products with antidiabetic potentials” was commissioned by the editorial office without any funding or sponsorship. LSC served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhat R, Karim AA. Tongkat Ali (Eurycoma longifolia Jack): a review on its ethnobotany and pharmacological importance. Fitoterapia 2010;81:669-79. [Crossref] [PubMed]
- Athimulam A, Kumaresan S, Foo DCY, et al. Modelling and optimisation of Eurycoma longifolia water extract production. Food Bioprod Process 2006;84:139-49. [Crossref]
- Kardono LB, Angerhofer CK, Tsauri S, et al. Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia. J Nat Prod 1991;54:1360-7. [Crossref] [PubMed]
- Tada H, Yasuda F, Otani K, et al. New antiulcer quassinoids from Eurycoma longifolia. European J Med Chem 1991;26:345-9. [Crossref]
- Kuo PC, Damu AG, Lee KH, et al. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg Med Chem 2004;12:537-44. [Crossref] [PubMed]
- Meng D, Li X, Han L, et al. Four new quassinoids from the roots of Eurycoma longifolia Jack. Fitoterapia 2014;92:105-10. [Crossref] [PubMed]
- Park S, Nhiem NX, Kiem PV, et al. Five new quassinoids and cytotoxic constituents from the roots of Eurycoma longifolia. Bioorg Med Chem Lett 2014;24:3835-40. [Crossref] [PubMed]
- Yusuf H, Satria D. Zulkarnain. The activity of eurycomanone derivatives on cancer cell lines. Int J Pharm Sci Res 2019;10:2947-50.
- Chan KL, O'Neill MJ, Phillipson JD, et al. Plants as sources of antimalarial drugs. Part 3. Eurycoma longifolia. Planta Med 1986;105-7. [Crossref] [PubMed]
- Morita H, Kishi E, Takeya K, et al. Highly oxygenated quassinoids from Eurycoma longifolia. Phytochem 1993;33:691-6. [Crossref]
- Itokawa H, Kishi E, Morita H, et al. Cytotoxic quassinoids and tirucallane-type triterpenes from the woods of Eurycoma longifolia. Chem Pharm Bull 1992;40:1053-5. [Crossref]
- Kuo PC, Shi LS, Damu AG, et al. Cytotoxic and antimalarial beta-carboline alkaloids from the roots of Eurycoma longifolia. J Nat Prod 2003;66:1324-7. [Crossref] [PubMed]
- Yusuf H, Satria D, Suryawati S, et al. Combination therapy of eurycomanone and doxorubicin as anticancer on T47D and MCF-7 cell lines. Sys Rev Pharm 2020;11:335-41.
- Muhamad S, Pihie L, Latif J, et al. Induction of apoptosis in MCF-7 via the Caspase pathway by longilactone from Eurycoma longifolia Jack. Res Pharm Biotechnol 2011;3:1-10.
- Cheah SC, Pihie A. Eurycomanone exerts antiproliferative activity via apoptosis upon MCF-7 cells. Available online: http://eprints.usm.my/42144/
- Tee TT, Azimahtol HL. Induction of apoptosis by Eurycoma longifolia jack extracts. Anticancer Res 2005;25:2205-13. [PubMed]
- Mulyati GD, Nurani LH, Widyarini S. Effects of co-chemotherapy ethyl acetate fraction of Eurycoma longifolia Jack roots and doxorubicin against apoptosis through expression p53 mutant and Bcl-2. Indonesian J Med Health 2017;8:68-77. [Crossref]
- Tung NH, Uto T, Hai NT, et al. Quassinoids from the Root of Eurycoma longifolia and Their Antiproliferative Activity on Human Cancer Cell Lines. Pharmacogn Mag 2017;13:459-62. [Crossref] [PubMed]
- Al-Salahi OS, Ji D, Majid AM, et al. Anti-tumor activity of Eurycoma longifolia root extracts against K-562 cell line: in vitro and in vivo study. PLoS One 2014;9:e83818 [Crossref] [PubMed]
- Wong PF, Cheong WF, Shu MH, et al. Eurycomanone suppresses expression of lung cancer cell tumor markers, prohibitin, annexin 1 and endoplasmic reticulum protein 28. Phytomedicine 2012;19:138-44. [Crossref] [PubMed]
- Zakaria Y, Rahmat A, Pihie AH, et al. Eurycomanone induce apoptosis in HepG2 cells via up-regulation of p53. Cancer Cell Int 2009;9:16. [Crossref] [PubMed]
- Morita H, Kishi E, Takeya K, et al. New quassinoids from the roots of Eurycoma longifolia. Chem Lett 1990;19:749-52. [Crossref]
- Ang HH, Chan KL, Mak JW. Effect of 7-day daily replacement of culture medium containing Eurycoma longifolia Jack constituents on the Malaysian Plasmodium falciparum isolates. J Ethnopharmacol 1995;49:171-5. [Crossref] [PubMed]
- Chan KL, Choo CY, Abdullah NR, et al. Antiplasmodial studies of Eurycoma longifolia Jack using the lactate dehydrogenase assay of Plasmodium falciparum. J Ethnopharmacol 2004;92:223-7. [Crossref] [PubMed]
- Kavitha N, Noordin R, Chan KL, et al. Cytotoxicity activity of root extract/fractions of Eurycoma longifolia Jack root against vero and Hs27cells. J Med Plants Res 2010;4:2383-7.
- Hajjouli S, Chateauvieux S, Teiten MH, et al. Eurycomanone and eurycomanol from Eurycoma longifolia Jack as regulators of signaling pathways involved in proliferation, cell death and inflammation. Molecules 2014;19:14649-66. [Crossref] [PubMed]
- Tran TV, Malainer C, Schwaiger S, et al. NF-κB inhibitors from Eurycoma longifolia. J Nat Prod 2014;77:483-8. [Crossref] [PubMed]
- Hien DTT, Long TP, Thao TP, et al. Anti-inflammatory effects of alkaloid enriched extract from roots of Eurycoma longifolia Jack. Asian Pac J Trop Biomed 2019;9:18-23. [Crossref]
- Ngoc PB, Pham TB, Nguyen HD, et al. A new anti-inflammatory β-carboline alkaloid from the hairy-root cultures of Eurycoma longifolia. Nat Prod Res 2016;30:1360-5. [Crossref] [PubMed]
- Hendra P. Evaluation of anthyperlipidemic, anti-inflammatory, and analgesic activities of Eurycoma longifolia in animal models. Int J Pharm Pharm Sci 2017;9:166-9. [Crossref]
- Husen R, Pihie AH, Nallappan M. Screening for antihyperglycaemic activity in several local herbs of Malaysia. J Ethnopharmacol 2004;95:205-8. [Crossref] [PubMed]
- Tsai CH, Fang TC, Liao PL, et al. The Powdered Root of Eurycoma longifolia Jack Improves Beta-Cell Number and Pancreatic Islet Performance through PDX1 Induction and Shows Antihyperglycemic Activity in db/db Mice. Nutrients 2020;12:2111. [Crossref] [PubMed]
- Nik Nur Shamiha ND, Siti Shukriyah S, Ryuichiro S, et al. Study on biological active components of Eurycoma Longifolia. Proceedings of BROMO Conference (BROMO 2018):305-9.
- Yusuf H, Hasballah K, Fitri Jamil K. Activity of ethyl acetate fractions of Tongkat Ali roots (Eurycoma longifolia, Jack) on blood glucose levels in Streptozotocin induced diabetic rats. Int J Pharm Sci Res 2019;10:2290-3.
- Fransisca GEK, Saptasari DC, Hendra P. The effect of pasak bumi roots towards blood glucose level in glucose-loaded mice. J Farm Sains Komun 2018;15:1-6. [Crossref]
- Chen Y, Phang WM, Mu AK, et al. Decreased expression of alpha-2-HS glycoprotein in the sera of rats treated with Eurycoma longifolia extract. Front Pharmacol 2015;6:211. [Crossref] [PubMed]
- Lahrita L, Kato E, Kawabata J. Uncovering potential of Indonesian medicinal plants on glucose uptake enhancement and lipid suppression in 3T3-L1 adipocytes. J Ethnopharmacol 2015;168:229-36. [Crossref] [PubMed]
- Ramli R, Khamis MF, Shuid AN. Bone micro-CT Assessments in an orchidectomised rat model supplemented with Eurycoma longifolia. Evid Based Complement Altern Med 2012;2012:Article ID 501858.
- Tee BH, Hoe SZ, Cheah SH, et al. First Report of Eurycoma longifolia Jack Root Extract Causing Relaxation of Aortic Rings in Rats. Biomed Res Int 2016;2016:1361508 [Crossref] [PubMed]
- Balan D, Chan KL, Murugan D, et al. Antiadipogenic effects of a standardized quassinoids-enriched fraction and eurycomanone from Eurycoma longifolia. Phytother Res 2018;32:1332-45. [Crossref] [PubMed]
- Lahrita L, Hirosawa R, Kato E, et al. Isolation and lipolytic activity of eurycomanone and its epoxy derivative from Eurycoma longifolia. Bioorg Med Chem 2017;25:4829-34. [Crossref] [PubMed]
- Rehman SU, Choe K, Yoo HH. Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence-Based Pharmacology and Toxicology. Molecules 2016;21:331. [Crossref] [PubMed]
- Ang HH, Sim MK. Eurycoma longifolia Jack enhances libido in sexually experienced male rats. Exp Anim 1997;46:287-90. [Crossref] [PubMed]
- Ang HH, Sim MK. Eurycoma longifolia increases sexual motivation in sexually naive male rats. Arch Pharm Res 1998;21:779-81. [Crossref] [PubMed]
- Ang HH, Sim MK. Eurycoma longifolia JACK and orientation activities in sexually experienced male rats. Biol Pharm Bull 1998;21:153-5. [Crossref] [PubMed]
- Ang HH, Lee KL. Effect of Eurycoma longifolia Jack on libido in middle-aged male rats. J Basic Clin Physiol Pharmacol 2002;13:249-54. [Crossref] [PubMed]
- Ang HH, Ngai TH, Tan TH. Effects of Eurycoma longifolia Jack on sexual qualities in middle aged male rats. Phytomedicine 2003;10:590-3. [Crossref] [PubMed]
- Ang HH, Lee KL, Kiyoshi M. Eurycoma longifolia Jack enhances sexual motivation in middle-aged male mice. J Basic Clin Physiol Pharmacol 2003;14:301-8. [Crossref] [PubMed]
- Ang HH, Lee KL, Kiyoshi M. Sexual arousal in sexually sluggish old male rats after oral administration of Eurycoma longifolia Jack. J Basic Clin Physiol Pharmacol 2004;15:303-9. [Crossref] [PubMed]
- Ang HH, Cheang HS, Yusof AP. Effects of Eurycoma longifolia Jack (Tongkat Ali) on the initiation of sexual performance of inexperienced castrated male rats. Exp Anim 2000;49:35-8. [Crossref] [PubMed]
- Ang HH, Cheang HS. Effects of Eurycoma longifolia jack on laevator ani muscle in both uncastrated and testosterone-stimulated castrated intact male rats. Arch Pharm Res 2001;24:437-40. [Crossref] [PubMed]
- Ang HH, Ngai TH. Aphrodisiac evaluation in non-copulator male rats after chronic administration of Eurycoma longifolia Jack. Fundam Clin Pharmacol 2001;15:265-8. [Crossref] [PubMed]
- Zanoli P, Zavatti M, Montanari C, et al. Influence of Eurycoma longifolia on the copulatory activity of sexually sluggish and impotent male rats. J Ethnopharmacol 2009;126:308-13. [Crossref] [PubMed]
- Heinrich M, Appendino G, Efferth T, et al. Best practice in research - Overcoming common challenges in phytopharmacological research. J Ethnopharmacol 2020;246:112230 [Crossref] [PubMed]
- Waldinger MD, Berendsen HH, Blok BF, et al. Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res 1998;92:111-8. [Crossref] [PubMed]
- Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med 2004;1:35-9. [Crossref] [PubMed]
- Berookhim BM, Bar-Chama N. Medical implications of erectile dysfunction. Med Clin North Am 2011;95:213-21. [Crossref] [PubMed]
- Udani JK, George AA, Musthapa M, et al. Effects of a Proprietary Freeze-Dried Water Extract of Eurycoma longifolia (Physta) and Polygonum minus on Sexual Performance and Well-Being in Men: A Randomized, Double-Blind, Placebo-Controlled Study. Evid Based Complement Alternat Med 2014;2014:179529 [Crossref] [PubMed]
- Ismail SB, Wan Mohammad WM, George A, et al. Randomized Clinical Trial on the Use of PHYSTA Freeze-Dried Water Extract of Eurycoma longifolia for the Improvement of Quality of Life and Sexual Well-Being in Men. Evid Based Complement Alternat Med 2012;2012:429268 [Crossref] [PubMed]
- Thu HE, Mohamed IN, Hussain Z, et al. Eurycoma Longifolia as a potential adoptogen of male sexual health: a systematic review on clinical studies. Chin J Nat Med 2017;15:71-80. [Crossref] [PubMed]
- Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia 2012;44:226-30. [Crossref] [PubMed]
- Tajul Ariff AS, Soelaiman IN, Pramanik J, et al. Effects of Eurycoma longifolia on Testosterone Level and Bone Structure in an Aged Orchidectomised Rat Model. Evid Based Complement Alternat Med 2012;2012:818072 [Crossref] [PubMed]
- Talbott SM, Talbott JA, George A, et al. Effect of Tongkat Ali on stress hormones and psychological mood state in moderately stressed subjects. J Int Soc Sports Nutr 2013;10:28. [Crossref] [PubMed]
- Tambi MI, Imran MK. Eurycoma longifolia Jack in managing idiopathic male infertility. Asian J Androl 2010;12:376-80. [Crossref] [PubMed]
- WHO laboratory manual for the Examination and processing of human semen, Part 1, Semen Analysis, fifth edition, World Health Organization, Geneva Switzerland, 2010.
- Jiwajinda S, Santisopasri V, Murakami A, et al. In vitro anti-tumor promoting and anti-parasitic activities of the quassinoids from Eurycoma longifolia, a medicinal plant in Southeast Asia. J Ethnopharmacol 2002;82:55-8. [Crossref] [PubMed]
- Chuen CS, Pihie AHL. [BIO04] Eurycomanone exerts antiproliferative activity via apoptosis upon MCF-7 cells. Available online: http://web.usm.my/nsf/proceedings/pBIO04.pdf
- Mahfudh N, Pihie AHL. Eurycomanone induces apoptosis through the up-regulation of p53 in human cervical carcinoma cells. J Cancer Mol 2008;4:109-15.
- Tee TT, Cheah YH, Hawariah LP. F16, a fraction from Eurycoma longifolia jack extract, induces apoptosis via a caspase-9-independent manner in MCF-7 cells. Anticancer Res 2007;27:3425-30. [PubMed]
- Tong KL, Chan KL, AbuBakar S, et al. The in vitro and in vivo anti-cancer activities of a standardized quassinoids composition from Eurycoma longifolia on LNCaP human prostate cancer cells. PLoS One 2015;10:e0121752 [Crossref] [PubMed]
- Katib S, Ruangrungsi N, Chaijaroenkul W, et al. Standardization parameters, Internal Transcribed Spacer nucleotide sequence and their anti-malarial activity of Eurycoma longifolia Jack. Int J Adv Pharm Biol Chem 2015;4:1-6.
- Hout S, Chea A, Bun SS, et al. Screening of selected indigenous plants of Cambodia for antiplasmodial activity. J Ethnopharmacol 2006;107:12-8. [Crossref] [PubMed]
- Wernsdorfer WH, Ismail S, Chan KL, et al. Activity of Eurycoma longifolia root extract against Plasmodium falciparum in vitro. Wien Klin Wochenschr 2009;121:23-6. [Crossref] [PubMed]
- Mohd Ridzuan MA, Noor Rain A, Zhari I, et al. Effect of Eurycoma longifolia extract on the Glutathione level in Plasmodium falciparum infected erythrocytes in vitro. Trop Biomed 2005;22:155-63. [PubMed]
- Low BS, Teh CH, Yuen KH, et al. Physico-chemical effects of the major quassinoids in a standardized Eurycoma longifolia extract (Fr 2) on the bioavailability and pharmacokinetic properties, and their implications for oral antimalarial activity. Nat Prod Commun 2011;6:337-41. [Crossref] [PubMed]
- Mohd Effendy N, Mohamed N, Muhammad N, et al. Eurycoma longifolia: Medicinal Plant in the Prevention and Treatment of Male Osteoporosis due to Androgen Deficiency. Evid Based Complement Alternat Med 2012;2012:125761 [Crossref] [PubMed]
- Thu HE, Mohamed IN, Hussain Z, et al. Exploring molecular mechanism of bone-forming capacity of Eurycoma longifolia: Evidence of enhanced expression of bone-related biomarkers. J Ayurveda Integr Med 2018;9:272-80. [Crossref] [PubMed]
- Thu HE, Hussain Z, Mohamed IN, et al. Eurycoma longifolia, a promising suppressor of RANKL-induced differentiation and activation of osteoclasts: An in vitro mechanistic evaluation. J Ayurveda Integr Med 2019;10:102-10. [Crossref] [PubMed]
- Li CH, Liao JW, Liao PL, et al. Evaluation of acute 13-week subchronic toxicity and genotoxicity of the powdered root of Tongkat Ali (Eurycoma longifolia Jack). Evid Based Complement Altern Med 2013;2013:Article ID 102987.
- Chan KL, Choo CY. The toxicity of some quassinoids from Eurycoma longifolia. Planta Med 2002;68:662-4. [Crossref] [PubMed]
- Bhat IA, Rathor PK, Mir IN, et al. Toxicological evaluation and effective dose selection of eurycomanone, a quassinoid of Eurycoma longifolia plant in fishes. Aquacul 2017;481:94-102. [Crossref]
Cite this article as: Segaran A, Chua LS, Mohd Ismail NI. A narrative review on pharmacological significance of Eurycoma longifolia jack roots. Longhua Chin Med 2021;4:35.

