The safety of Chinese herbal medicine: a systematic review of adverse events in randomized controlled trials
Introduction
Traditional Chinese medicine (TCM) is centuries old, has developed a unique system of diagnosis and treatment of disease in practice, and is not only widely used in China, but is also practiced in other Asian countries and Western countries as well (1,2). Chinese herbal medicine (CHM) is the mainstay and principal form of TCM practice, which comprises a pharmacopoeia of thousands of medicinal substances, primarily plants and some minerals and animal substances (3). There are several administration routes of CHM, including oral intake, inhalation, external use, intravenous injection, intramuscular injection, and other means. Of these, oral administration is the main and traditional administration route of CHM.
Oral CHM has been clinically used for thousands of years, and generally speaking, oral CHM is believed to cause fewer side effects as it is generally extracted from natural products without artificial additives (4,5), although some Chinese herbs have been known to be toxic to humans. In 1964, two cases of acute renal failure caused by a large dose of mutong were reported, which were the first case reports on CHM in China (6). Aristolochic acid, which is isolated from Aristolochia debilis Sieb. et Zucc. (known as madouling in CHM), can lead to “Chinese herb nephropathy”, a rapidly progressive interstitial fibrosis of the kidney, as was seen in a group of Belgian women who had all followed a slimming regimen (7). Glycyrrhiza spp. (liquorice root) and its extracts, contain mineralocorticoid, which can cause adverse reactions such as edema, hypertension, and electrolyte imbalances (8).
Currently, the safety of CHM is an important consideration in decision-making for patients, clinicians, policy makers, and regulators. The incidence of adverse events (AEs) is one of the measures used to observe and study the safety of interventions. An AE is defined as “any untoward medical occurrence in a patient or clinical investigation subject administered a treatment, that does not necessarily have a causal relationship with this treatment” (9). Reporting AEs during clinical trials presents a vital component of assessing an intervention’s safety.
Randomized controlled trials (RCTs) are recommended as the gold standard to prove the efficacy of CHM, which also offer an excellent opportunity to evaluate harms of CHM using the most robust experimental design (10), especially when lacking large scale observational studies and national monitoring data for the safety of CHM (11,12). In recent years, the number of CHM RCTs has increased gradually, and thus systematically evaluating the safety of CHM in the body of published RCTs is urgently needed.
However, to our knowledge, such a review has not yet been performed. Therefore, the purpose of this article was to systematically review and analyze the incidence of AEs in CHM RCTs. Through this systemic review, greater attention is expected to be paid to the study of AEs in RCTs in order to promote a more healthy and positive development and application of CHM.
We present the following article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (available at http://dx.doi.org/10.21037/lcm-20-23).
Methods
Literature search
Using “randomized controlled trial”, “controlled clinical trial”, “randomized”, “randomised”, “clinical trial”, “traditional Chinese medicine”, “Chinese herbal drugs”, “oriental traditional medicine” and “medicinal plants”, etc. as search words, we searched the titles and abstracts in three electronic databases including MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases. Searches were restricted to RCTs of CHM published in the English language between January 2010 and December 2019. The full MEDLINE search strategy is available in Figure S1.
Inclusion and exclusion criteria
We included studies that were (I) RCTs focusing on oral CHM, both as single-use or in combination with other interventions; (II) and focusing on any disease or for any comparison. We excluded the following: (I) other TCM interventions, like acupuncture, moxibustion, massage, guasha, cupping, tai chi, qi gong, etc.; (II) other routes of CHM, like spray, washing, ointment, iontophoretic injection, etc.; (III) phase I or pharmacokinetics trials; (IV) self-described preliminary or pilot studies; (V) follow-up or secondary analysis of data; (VI) protocols or conference abstracts; (VII) use for healthy subjects.
We defined CHM using the criteria from our previous study (13). CHMs are preparations derived from plants or parts of plants (e.g., leaves, stems, buds, flowers, roots, or tubers) that grow in China and have been widely used for medical purposes. CHMs include single herbs (or extracts from single herbs) and compound formulas of several herbs in all forms of preparation formulation (e.g., oral liquid, tablet, capsule, pill, granule, and decoction). Studies focusing on Japanese herbal medicine, Korean herbal medicine, or other countries were excluded. Plant-derived chemicals or synthetic chemicals which contain constituents of plants were also excluded.
Selection of studies
Because of the large number of CHM RCTs published in English from 2010 to 2019, we used the random sampling method and proportion used in other studies (14-16). SAS for Windows (version 9.4; Order Number: 9C1XJD) was used to generate a 20% random sampling number table. We numbered and sorted all the retrieved articles; the numbers on the number table was corresponded to the number of total articles.
The RCTs selected for our study were assigned to two teams (two reviewers in each team). All reviewers (Y Ha, X Wang, R Zhang and C Wei) individually and independently screened the titles and abstracts of a 20% sample to determine those articles potentially related to our study. Based on this first assessment, we then obtained the full text of these articles, and the same two reviewers independently reviewed all these potentially eligible studies to find studies that fulfilled the inclusion criteria. Any disagreement in study selection was resolved by consensus or by discussion with a third reviewer (J Hu).
Data extraction and quality assessment
Two teams (two reviewers in each team, Y Ha, X Wang, R Zhang and C Wei) independently extracted data using a standard data extraction form that contained the following fields: (I) publication details, including publication year, journal name, and publication title; (II) characteristics of study participants, including disease and age; (III) intervention information, including interventions in treatment and control groups, and treatment duration; (IV) safety information. For the most of included RCTs, information for the judgement of causal inference from AEs to adverse drug reactions (ADRs) was insufficient. We were thus unable to distinguish ADRs from AEs, so all the safety information reported was considered to be AEs. We used WHO Adverse Reaction Terminology (WHOART) system organ class 2015 to standardize AEs.
The trial quality was assessed by two teams (two reviewers in each team: Y Ha, X Wang, R Zhang and C Wei) for each study separately using the following five domains of the Cochrane Risk-of-Bias Tool (17): (I) random sequence generation (i.e., selection bias), (II) allocation concealment (i.e., selection bias), (III) blinding of participants and personnel (i.e., performance bias), (IV) blinding of outcome assessment (i.e., detection bias), and (V) incomplete outcome data. Authors resolved disagreement by consensus, and another review author (J Hu) was consulted to resolve any disagreements.
Statistical analysis
OpenMeta [Analyst] software was performed to meta-analysis and subgroup analyses. Incidence of AEs with its 95% confidence interval (CI) was calculated. Subgroup analyses were performed based on four factors: dosage form of CHM; disease classification according to the International Classification of Disease revision 10 (ICD-10); single use of CHM or in combination with Western medicine (WM); administration to elderly, child, or adult participants.
Results
Flow of included studies
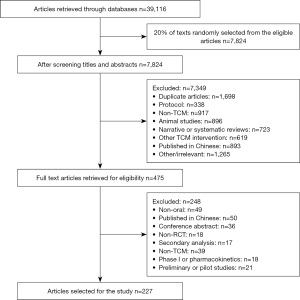
A total of 39,116 articles were identified, and we randomly selected 20% (n=7,824) from the eligible articles. After the titles and abstracts were screened, 475 articles were eligible. Full texts of these 475 articles were retrieved, and 227 RCTs were included in our study. Details of the study screening process can be seen in Figure 1.
Risk of bias assessment
Of the 227 RCTs, 142 (62.6%) used the adequate random sequence generation method, while 100 (44.1%) used adequate allocation concealment. Furthermore, 92 (40.5%) studies blinded participants and key study personnel, and 24 (10.6%) blinded outcome assessors. Incomplete outcome data in 149 (65.6%) RCTs were adequately addressed.
Analysis of AEs
A total of 57 (25.1%) of the 227 RCTs did not report any AEs. In the remaining 170 RCTs, considering that some RCTs used CHM both in the intervention group and control group, it was necessary to analyze all patients using CHM as statistical analysis units when calculating the incidence of AEs. Of the 170 RCTs, 21 used CHM in 2 groups, and 2 used CHM in 3 groups. Thus, we analyzed the 195 CHM groups in our study.
Of the 195 CHM groups, 22 (11.3%) included information for the total number of AEs, but not for specific AE type, and they were not included into our analysis. Additionally, 61 groups (including 5,572 patients) had no AEs, and when we analyzed the incidence of AEs, 5,572 patients were included in the denominator.
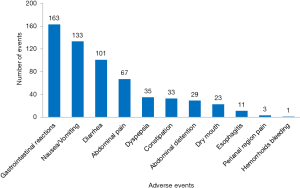
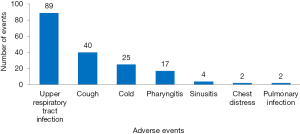
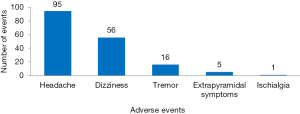
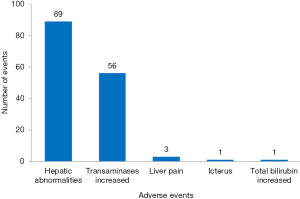
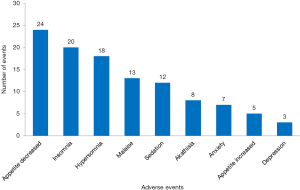
There were 1,765 AEs in 112 (including 10,754 patients) CHM groups, and the total incidence of AEs was 10.8% (95% CI: 10.3%, 11.3%). According to the WHOART system organ class, the top 5 most common classes were gastrointestinal disorders (33.94%), respiratory disorders (10.14%), neurological disorders (9.80%), liver and biliary disorders (8.50%), and psychiatric disorders (6.23%) (Table 1). We listed the specific AEs of these five classes. The most common AEs were gastrointestinal reactions, nausea/vomiting and diarrhea in gastrointestinal disorders (Figure 2), upper respiratory tract infection, cough and cold in respiratory disorders (Figure 3), headache, dizziness and tremor in neurological disorders (Figure 4), hepatic abnormalities, transaminase increase and liver pain in liver and biliary disorders (Figure 5), appetite decrease, insomnia and hypersomnia in psychiatric disorders (Figure 6).

Full table
Subgroup analyses of AEs
Of the seven dosage forms, the tablet form showed the highest AE incidence (14.0%, 95% CI: 12.6%, 15.4%), followed by capsules (12.4%, 95% CI: 11.1%, 13.7%). Liquids showed the lowest AE incidence (2.8%, 95% CI: 1.2%, 4.5%), followed by decoctions (6.3%, 95% CI: 5.4%, 7.1%) (Table 2).

Full table
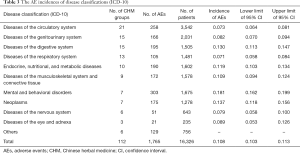
The highest AE incidence was mental and behavioral disorders (18.1%, 95% CI: 16.2%, 19.9%), followed by neoplasms (13.7%, 95% CI: 11.8%, 15.6%). Treatments for diseases of the circulatory system (7.3%, 95% CI: 6.4%, 8.1%) and nervous system (7.9%, 95% CI: 5.8%, 10.0%) had the lowest AE incidences (Table 3).
Full table
The AE incidence in the CHM in combination with WM group (13.1%, 95% CI: 10.6%, 15.5%) was higher than that in the CHM group (10.9%, 95% CI: 10.3%, 11.4%) and CHM in combination with simulators of WM group (9.7%, 95% CI: 8.4%, 10.9%) (Table 4).

Full table
The AE incidence of CHM for child (12.9%, 95% CI: 11.0%, 14.8%) and elderly participants (11.2%, 95% CI: 5.5%, 16.9%) was higher than that of adult participants (10.6%, 95% CI: 10.1%, 11.1%) (Table 5).

Full table
Discussion
Guideline developers, policy makers, and clinicians need to balance the safety and effectiveness of interventions in RCTs when making clinical decisions. One of the primary goals of a clinical trial is to assess treatment safety, which is needed alongside efficacy information for a trial to be clinically informative (18). A major way to assess safety is through the reporting of AEs that arise during trials. Incomplete AE reporting can lead to the underestimation of risk, potentially compromising regulatory approval and informed clinical use of treatments (19).
Past research on AE reporting practices in CHM RCTs published in Chinese language identified many reporting inadequacies. For example, we did a systematic review of nearly 700 clinical studies published in the Chinese language to analyze the incidence of AEs in ginkgo biloba leaf extract injection, and 65.9% of these studies did not report any AEs (20). Some researchers have shown that the methodology and reporting quality of CHM RCTs published in English is evidently higher than those published in Chinese language (21,22).
The incidence of AEs is challenging to calculate as it requires adequate reporting of AEs in clinical trials (e.g., number and nature of events). Therefore, in this systematic review, we included the CHM RCTs which were published in English to analyze the incidence of AEs. Despite this, we found that 25.1% of these RCTs did not report any AEs, while 11.3% reported the total number of AEs, but did not report the specific AEs, so we did not include these into our analysis. Other studies have shown that the reporting rate of AEs is low in CHM trials (23,24).
There are many advantages of reporting AEs in RCTs; for example, reports of previously unknown interactions will be helpful for safer use of the drugs in the future; providing evidence of AEs for systematic review; and identifying risk factors for AEs, such as age or gender. Therefore, correctly and clearly reporting the complete information about ADR/AEs is very important. The Consolidated Standards of Reporting Trials (CONSORT) extension of harms was developed in 2004. It standardizes AE reporting by creating a 10-item checklist of essential AE information for trial publication (25). AE data in future RCTs of CHM should be reported in accordance with the CONSORT extension of harms.
This study conducted a meta-analysis and found that the total incidence of AEs in the included RCTs of oral CHM was 10.8% (95% CI: 10.3%, 11.3%). According to WHOART system-organ class, CHM can lead to AEs in various tissues and systems. One-third of AEs were gastrointestinal disorders, which mainly presented as gastrointestinal reactions, nausea/vomiting, and diarrhea, perhaps because oral CHM directly stimulates the gastrointestinal tract, easily causing AEs in the digestive system. The next most common AEs were respiratory disorders, neurological disorders, liver and biliary disorders, and psychiatric disorders. Similar results have also been reported in other studies (26-28). It is recommended that clinicians pay attention to the AEs that may occur in related systems, and AEs should be strictly monitored, especially when patients have these diseases.
Of the seven dosage forms, the highest AE incidences occurred with the use of tablets and capsules, while the lowest incidences occurred with liquids and decoctions. Huang et al.’s (28) and Qiao et al.’ studies (29) also indicated that the highest AE incidences occurred with tablets and capsules forms. CHM currently includes several dosage forms, among which decoction is the most traditionally used form, having been used for thousands of years with fewer AEs. Chinese patent medicines (zhongchengyao), as a part of CHM, may come in the form of tablets, pills, or capsules. Chinese patent medicines generally are made with more modern pharmaceutical technology consisting of several herbs and other ingredients, which may cause side-effects.
Our systematic review found that the incidence of AEs in CHM in combination with WM groups was higher than that of the non-WM use group. It is widely acknowledged that combined CHM and WM can improve the clinical efficacy in the treatment of some conditions such as hypertension, cancer, and depression (30). However, CHM and WM, after all, belong to two different systems of medicine. The theories that underlie each are distinct, and so their combination entails significant complexity (31). In addition, most CHMs are complex mixtures of more than one active ingredient, which may increase the possibilities of interactions between CHM and WM (31), with these interactions sometimes leading to unexpected side-effects. To avoid this, information regarding drug-herb interactions and contradictions should be made widely available for medical practitioners and pharmacists through various methods (e.g., drug-herb interaction database, textbooks, education services, training, etc.), so practitioners can be made aware of and avoid potential interactions between CHM and WM.
In our study, the AE incidence of CHM for child and elderly participants were higher than that for adults participants. The functions of organs have not yet matured in children and gradually decline in the elderly, and so children and the elderly are weaker in drug tolerance and metabolic capacity than adults (5,32,33). In addition, concerning the medicinal instructions for CHM, many CHM products do not indicate the recommended dosage for children, or only indicate that the dosage should be reduced for children. This may lead to different clinicians using different dosages of CHM for children which may increase the risk of adverse AEs. It is recommended that clinicians calculate the dosage by the weight, age, or body surface area of children when making treatment decisions.
There are several limitations to our study. Firstly, in the included RCTs, the attempts to judge causal inference from AEs to ADRs were insufficient. We were thus unable to distinguish ADRs from AEs, and so all the safety information reported was considered to be AEs. Secondly, since 24.7% of the RCTs did not report any safety data and 11.3% only reported the total number of AEs but not the specific AE type, we did not include these into our analysis; therefore, the actual incidence of AEs might have been underestimated. Thirdly, we used a random sampling method performed in other studies and randomly selected 20% of the total studies for analysis; as a consequence, the findings of our study can only be considered an approximation.
Conclusions
Overall, CHM can lead to AEs in various tissues and systems. The most common AEs in our review were gastrointestinal disorders, including gastrointestinal reactions, nausea/vomiting and diarrhea, and others. Of the seven dosage forms, the highest AE incidences occurred with use of tablets and capsules, while the lowest AE incidences occurred with liquids and decoctions form. The incidence of AEs in CHM in combination with the WM groups was higher than that of the non-WM use group, while child and elderly participants had a higher AE incidence than did adult participants. Future clinical trials of CHM should provide greater details relating to AEs in order to more accurately inform clinical practice.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81973694 and 81774146) and the “13th Five-Year” National Science and Technology Major Project for New Drugs (No: 2019ZX09734001). The funders had no role in study design, decision to publish, or preparation of the manuscript.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Longhua Chinese Medicine for the series “Narrative & Evidence-based Medicine for Traditional Medicine: from basic research to clinical practice and trail”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/lcm-20-23
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-23). The series “Narrative & Evidence-based Medicine for Traditional Medicine: from basic research to clinical practice and trail” was commissioned by the editorial office without any funding or sponsorship. BL served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang WJ, Zhang T. Integration of traditional Chinese medicine and Western medicine in the era of precision medicine. J Integr Med 2017;15:1-7. [Crossref] [PubMed]
- Rashrash M, Schommer JC, Brown LM. Prevalence and predictors of herbal medicine use among adults in the United States. J Patient Exp 2017;4:108-13. [Crossref] [PubMed]
- Zhu Y. Chinese materia medica: chemistry, pharmacology and applications. Amsterdam, The Netherlands: Harwood Academic, 1998.
- Chan K, Zhang H, Lin Z. An overview on adverse drug reactions to traditional Chinese medicines. Br J Clin Pharmacol 2015;80:834-43. [Crossref] [PubMed]
- Zeng ZP, Jiang J. Analysis of the adverse reactions induced by natural product-derived drugs. Br J Pharmacol 2010;159:1374-91. [Crossref] [PubMed]
- Chen R. Adverse drug reaction and post-marketing evaluation of Chinese herbal medicine. Journal of Hubei University of Chinese Medicine 2003;5:5-8.
- Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int 2008;74:158-69. [Crossref] [PubMed]
- Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhizasp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol 2006;46:167-92. [Crossref] [PubMed]
- National Institute on Deafness and Other Communication Disorders, Reporting Adverse Events in NIDCD Clinical Trials. Available online: grants.nih.gov/grants/guide/notice-files/NIDCD_Adverse_Events.doc. Accessed December 31, 2019.
- Flower A, Witt C, Liu J, et al. Guidelines for randomised controlled trials investigating Chinese herbal medicine. J Ethnopharmacol 2012;140:550-4. [Crossref] [PubMed]
- Hu R, Golder S, Yang G, et al. Comparison of drug safety data obtained from the monitoring system, literature, and social media: An empirical proof from a Chinese patent medicine. PLoS One 2019;14:e0222077.
- Guo XJ, Ye X, Wang X, et al. Reporting patterns of adverse drug reactions over recent years in China: analysis from publications. Expert Opin Drug Saf 2015;14:191-8. [Crossref] [PubMed]
- Hu J, Zhang J, Zhao W, et al. Cochrane systematic reviews of Chinese herbal medicines: an overview. PLoS One 2011;6:e28696 [Crossref] [PubMed]
- Bhaloo Z, Adams D, Liu Y, et al. Primary Outcomes Reporting in Trials (PORTal): a systematic review of inadequate reporting in pediatric randomized controlled trials. J Clin Epidemiol 2017;81:33-41. [Crossref] [PubMed]
- Chevan J, Haskvitz EM. Reported characteristics of participants in physical therapy-related clinical trials. Phys Ther 2015;95:884-90. [Crossref] [PubMed]
- Pinto RZ, Elkins MR, Moseley AM, et al. Many randomized trials of physical therapy interventions are not adequately registered: a survey of 200 published trials. Phys Ther 2013;93:299-309. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726-32. [Crossref] [PubMed]
- Ioannidis JPA. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Arch Intern Med 2009;169:1737-9. [Crossref] [PubMed]
- Hu J, Xu R, Gao L, et al. Incidence of adverse drug events on extract of ginkgo biloba leaves injection: a meta-analysis. Chin J Pharmacoepidemiol 2017;26:339-44.
- Chen Y, Zeng X, Liu D, et al. Critical quality evaluation and application value of network Meta-analyses in traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2019;44:5322-8. [PubMed]
- Cui Z, Bian Y. Evaluation of methodological quality of traditional Chinese medicine clinical trials. Lishizhen Medicine and Materia Medica Research 2019;30:2302-4.
- Coyle M, Shergis JL, Liu S, et al. Safety of Chinese herbal medicine for chronic obstructive pulmonary disease. Evid Based Complement Alternat Med 2015;2015:380678 [Crossref] [PubMed]
- Zhou X, Li CG, Chang D, et al. Current status and major challenges to the safety and efficacy presented by Chinese herbal medicine. Medicines 2019;6:14. [Crossref] [PubMed]
- Ioannidis JP, Evans SJ, Gøtzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004;141:781-8. [Crossref] [PubMed]
- Sun L, Liu F, Chen W, et al. Evaluation analysis of traditional Chinese medicine adverse reaction system. Chinese Journal of Drug Evaluation 2016;33:155-64.
- Meng X, Cao R, Ye X, et al. Analysis of 256 cases of adverse reactions of traditional Chinese medicine. Clinical Journal of Traditional Chinese Medicine 2019;31:1853-6.
- Chen Y, Huang YS, Li J. Adverse drug reactions induced by Chinese patent medicine: analysis of 333 cases. Evaluation and Analysis of Drug-Use in Hospitals of China 2014;14:754-7.
- Qiao Y, Yun Y, Xu H, et al. Adverse drug reactions induced by Chinese patent medicine in Xijing hospital during 2014-2016: analysis of 256 cases. Chin J Pharmacoepidemiol 2016;25:708-10.
- Zhou X, Seto SW, Chang D, et al. Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol 2016;7:201. [Crossref] [PubMed]
- Zhang J, Onakpoya IJ, Posadzki P, et al. The safety of herbal medicine: from prejudice to evidence. Evid Based Complement Alternat Med 2015;2015:316706 [PubMed]
- Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther 2019;24:4-9. [Crossref] [PubMed]
- Rodieux F, Gotta V, Pfister M, et al. Causes and consequences of variability in drug transporter activity in pediatric drug therapy. J Clin Pharmacol 2016;56:S173-92. [Crossref] [PubMed]
(English Language Editor: J. Gray)
Cite this article as: Hu J, Zhang H, Feng S, Ha Y, Wei C, Wang X, Zhang R, Li B. The safety of Chinese herbal medicine: a systematic review of adverse events in randomized controlled trials. Longhua Chin Med 2020;3:7.