
The possible role of green tea on osteoarthritis: a narrative report
Introduction
Osteoarthritis (OA) is a chronic inflammatory condition that may affect millions of people around the world. Patients present synovial inflammation, articular cartilage degradation, and subchondral bone lesion. Several joints such as knees, hips, shoulders, and feet may present this condition (1,2).
OA is incurable and, therefore, treatment should be conducted for life. The conventional non-surgical therapies usually are based on the use of analgesics, nonsteroidal anti-inflammatory medications (NSAIDs), hyaluronic acid, and corticosteroids. Although these therapies can relieve symptoms, they are often associated with numerous adverse effects. NSAIDs and acetaminophen are associated with gastrointestinal and cardiovascular issues, primarily if they are used for long periods (3-5). Due to these reasons, there is an increasing interest in the use of medicinal plants for treating or preventing this inflammatory condition (6,7) and green tea (GT) has been considered as adjuvant therapy (8,9).
GT is one of the most popular beverages consumed worldwide and is produced with the leaves of Camellia sinensis. GT and its bioactive compounds have been associated with the prevention and treatment of several pathological conditions. The bioactive compounds found in this plant include a plethora of antioxidants, vitamins, and flavonoid-like polyphenols (10).
The main effects of GT on human health are related to the presence of the catechins epicatechin, epigallocatechin, epicatechin-gallate, and epigallocatechin-gallate (ECGC), which belong to a family of flavonoid-like polyphenols. These compounds can promote significant anti-inflammatory and antioxidant properties and can generate benefits in different conditions such as inflammatory diseases, obesity, diabetes, cardiovascular diseases, and cancer (11-14).
Since GT and its bioactive compounds can have critical anti-inflammatory effects, the purpose of this review was to show the effects of this plant on the therapeutic approach to OA. We present the following article in accordance with the Narrative Review checking checklist (available at http://dx.doi.org/10.21037/lcm-20-32).
Methods
Data sources
The databases MEDLINE-PubMed (National Library of Medicine, National Institutes of Health) and EMBASE were used to perform this review (from August 2000 to August 2020).
Focal question
This review was performed to answer the following focal question: Can green tea or its bioactive compounds bring benefits to the treatment of OA?
Search
Also following PRISMA guidelines (15), the combination of terms that were used for this search was osteoarthritis and Green Tea or Camellia sinensis or epicatechin or epigallocatechin, or epicatechingallate or epigallocatechingallate.
Results and discussion
We only find one clinical study that considered the use of GT or its bioactive compounds in the therapeutic approach of OA.
GT and its bioactive compounds
As mentioned before, GT leaves are rich in catechins, and the most relevant is epigallocatechin-3-gallate (ECGC), which corresponds to 30–60%. Epicatechin, epigallocatechin, and epicatechin-3-gallate correspond to 6%, 18–28%, and 8–12%, respectively. Altogether, these constituents represent about 30–36% of the dry weight of the leaves. A cup of GT approximately contains 60 to 125 mg catechins, and many of the beneficial health properties are attributed to the polyphenol EGCG. These benefits include anti-inflammatory, antioxidant, anti-diabetic, anti-obesity, anti-cancer, and neuroprotective effects. Indeed, authors have shown that EGCG is 25–100 times more potent than vitamin C and vitamin E as an antioxidant (14,16-19).
The antioxidant effects of GT polyphenols play important actions in inhibiting several signaling pathways related to inflammatory processes. The inhibition may affect IL-1, IL-6, IL-8, intercellular adhesion molecule-1 expression (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), TNFα, nuclear factor-kappa B (NF-κB) mediated I kappa B kinase complex (IKK), and cyclooxygenase-2 (Cox-2) (Figure 1) (20-22).
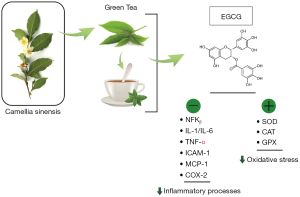
Furthermore, EGCG also shows antioxidant actions once it shows scavenger properties. The prevention of ROS production and consequent prevention of oxidative stress has a critical role in the inhibition of OA pathogenesis (Figure 1). EGCG can restore the enzymatic actions of glutathione peroxidase (GPX) and can regulate the levels of glutathione. Besides that, EGCG is capable of inhibiting the production of hydrogen peroxide and nitric oxide (12-14,16,23).
Some pathophysiologic aspects of OA
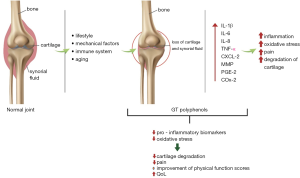
Usually, chondrocytes keep a dynamic equilibrium between production and degradation of the extracellular matrix, such as aggrecan and type II collagen, which are the most relevant proteoglycan in the articular cartilage. In the OA, there is a disorder of synovial joints presenting focal areas of articular cartilage damage, associated with new bone formation at the margin of the joints, modifications in the subchondral bone, synovitis, and thickening of the joint capsule. The results for these dysfunctions depend on the severity of the damage, but the characteristic clinical symptoms are inflammation, reduced range of motion, mild-to-severe pain, and deformity. In addition to the degradation of the articular cartilage, OA also involves damages to bone and synovium in the joints and is associated with lifestyle, diet, genetics, and aging (24,25).
The pathophysiology of OA is not fully understood, but it is known that the pathogenesis involves inflammatory processes, oxidative stress, osteoclastogenesis, and proteolytic degradation of cartilage (Figure 2). Chondrocytes are the only cells found in the cartilage matrix and are the central regulators of matrix catabolism and anabolism in the articular cartilage. In the presence of inductive stimuli, there is an increased activity or expression of cartilage proteinases, which are primarily synthesized by chondrocytes leading to OA (25).
Chondrocytes are also responsible for the production of pro-collagen N-proteinases, which may include disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-3. These proteinases are related to the removal of the N-terminal pro-peptide of type II pro-collagen, augmenting the cartilage matrix anabolism (26,27).
Increased catabolism of the cartilage matrix (cartilage collagen and aggrecan) results in the degeneration of articular cartilage in arthritic processes. MMPs (MMP-2 and MMP-13) and aggrecanases (ADAMTS-4 and ADAMTS-5) have been detected in OA (28,29).
Chondrocytes, osteoclasts, and stromal cells should also be mentioned once they produce the Receptor Activator Factor kβ Ligand (RANKL). The connection of RANK to RANKL in osteoclasts precursor cells leads to the activation of I Kappa B Kinase (IKK) and phosphorylation of I Kappa B alpha (IkBα) that are related to the activation of osteoclastogenesis. Moreover, in OA, interleukins such as IL-1β, IL-6, IL-8, IL-17, and TNF-α are associated with increased production of RANKL resulting in bone resorption (30-32).
This scenario of inflammation and increased levels of cytokines and proteases results in the death of chondrocytes and synoviocytes stimulation leading to the recruitment of mononuclear and polymorphonuclear factors collaborating to more cartilage destruction. In addition to the increase in pro-inflammatory cytokines and TNF-α, the presence of prostaglandin-2 (PGE-2) and free radicals such as reactive oxygen and nitrogen species are linked to the pathogenesis of the disease (33,34).
GT and OA
Fechtner et al. (22) showed the molecular mechanisms for the differences in the anti-inflammatory actions of epigallocatechin, epicatechingallate, and epigallocatechin-gallate in the culture of humans RASFs. They showed that there are different effects of the catechins on the IL-1β signal transduction pathways. The authors observed that Epigallocatechin, and EGCG promoted anti-inflammatory action in human RASFs due to the inhibition of IL-1β-induced mediators such as IL-6 and IL-8, COX-1/2), and MMP, which may work as the basis for anti-inflammatory actions attributed to the GT extracts.
The production of cytokines and MMP in human RASFs is directly associated with augmented levels of prostaglandins in response to IL-1β (35) and a study showed that EGCG inhibited the expression of TNF-α, and IL-1β-induced COX-2 in human OA chondrocytes (8,36).
EGCG is also related to the regulation of the expression of inducible nitric oxide synthase; it is capable of inhibiting IL-1β, induced phosphorylation, and degradation of IκBα to downregulate NFκB translocation (37). This catechin can also inhibit the expression of MMP-1 and MMP-13 and the catabolism of proteoglycan and type II collagen in human cartilage and can inhibit ADAMTS-1, ADAMTS-4, and ADAMTS-5 (24,38,39).
Rasheed et al. (40) investigated the effects of EGCG OA cartilage and found that this compound promoted the up-regulation of the expression of 19 miRNAs and the down-regulation in the expression of 17 miRNAs (the expression of 1311 miRNAs were unchanged) in IL-1β-stimulated OA chondrocytes of humans. EGCG led to the inhibition of the expression of IL-1β-induced ADAMTS-5 and led to the up-regulation in the expression of hsa-miR-140-3p. These results provided valuable insights into the molecular basis for the effects of EGCG in OA and indicated that this role in OA chondrocytes might be associated with its capacity to inhibit inflammatory pathways via modulation of miRNAs expressions.
Furthermore, EGCG can improve IL-1β-mediated suppression of the synthesis of transforming growth factor β, and can enhance the production of type II collagen and aggrecan core protein in articular chondrocytes (41).
A study performed by Comblain et al. (42) evaluated the effects of a mixture of GT, curcuminoids, and hydrolyzed collagen on the inflammatory and catabolic mediators in osteoarthritic human chondrocytes. Their results showed that about 4,000 genes were differentially expressed according to inflammation, cartilage catabolism, and angiogenesis. Moreover, they observed that the mixture promoted beneficial effects on OA physiopathology due to the regulation in the release of key catabolic and inflammatory factors. The authors postulated that these findings suggest that these natural ingredients could be used in the management of OA.
Comblain et al. (9) performed a randomized, double-blind, prospective, placebo-controlled study showing the effects of a diet supplemented with GT, curcuminoids extract and hydrolyzed collagen in owner’s dogs with OA. The inclusion criteria were the presence of clinical and radiographic signs of OA (presence of subchondral bone sclerosis and osteophytes) on at least one limb, and no evidence of systemic disease. After three months of treatment, animals that received the supplemented diet presented a significant decrease in pain that was not observed in the control group. The biomarkers of OA did not present statistical differences between the treated group and placebo.
Hashempur et al. (43) performed a randomized open-label active-controlled clinical trial with adults with knee OA that were allocated to receive the GT extract and diclofenac (treated group) or diclofenac alone (control group) for four weeks. They observed that the group that received the plant presented a significant reduction in the following scores: total WOMAC, WOMAC physical function scores, and VAS pain when compared to the control group. They did not see significant differences regarding WOMAC pain and stiffness scores between groups. No adverse effects were reported for patients included in the treated group. These results suggest that GT could be considered as a safe adjuvant in the treatment and control of pain, and for the improvement of knee joint physical function in subjects with OA.
For the reasons mentioned above, we suggest that GT and its bioactive compounds, mainly ECGC, could have a role in improving the severity of symptoms of OA (Figure 2).
Conclusions
Due to the impressive antioxidant and anti-inflammatory effects of this plant, we were surprised by the lack of studies that had used GT in subjects with OA. For these reasons, and since OA is a chronic, degenerative, painful, and inflammatory condition that affects millions of people around the globe (and the conventional treatments are associated with several adverse effects), we suggest that clinical trials, observational studies, and other studies with humans should be performed to evaluate the effects of this incredible plant or its components in the therapeutic approach or the prevention of OA.
Summary
GT and its bioactive compounds play an important anti-inflammatory role and can be used as a therapeutic adjuvant in inflammatory diseases. However, for OA, there are still very few clinical trials that show evidence of clinical improvement of the disease. For this reason, new studies must be performed so that doctors can consider this plant as an adjunct to treatment.
Acknowledgments
We acknowledge Renato Vono for drawing the figures (opa@vonodesigner.com).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/lcm-20-32
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-32). SMB serves as an unpaid editorial board member of Longhua Chinese Medicine from Jun 2020 to May 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li XZ, Zhang SN. Recent advance in treatment of osteoarthritis by bioactive components from herbal medicine. Chin Med 2020;15:80. [Crossref] [PubMed]
- Li D, Li S, Chen Q, et al. The Prevalence of Symptomatic Knee Osteoarthritis in Relation to Age, Sex, Area, Region, and Body Mass Index in China: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2020;7:304. [Crossref] [PubMed]
- Ershad M, Ameer MA, Vearrier D. StatPearls Publishing 2020;LLC:2020.
- Senthelal S, Li J, Goyal A, et al. StatPearls Publishing 2020;LLC:2020.
- Raeissadat SA, Gharooee Ahangar A, Rayegani SM, et al. Platelet-Rich Plasma-Derived Growth Factor vs Hyaluronic Acid Injection in the Individuals with Knee Osteoarthritis: A One Year Randomized Clinical Trial. J Pain Res 2020;13:1699-711. [Crossref] [PubMed]
- Yu G, Xiang W, Zhang T, et al. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complement Med Ther 2020;20:225. [Crossref] [PubMed]
- Leong DJ, Choudhury M, Hirsh DM, et al. Nutraceuticals: potential for chondroprotection and molecular targeting of osteoarthritis. Int J Mol Sci 2013;14:23063-85. [Crossref] [PubMed]
- Vaishya R, Agarwal AK, Shah A, et al. Current status of top 10 nutraceuticals used for Knee Osteoarthritis in India. J Clin Orthop Trauma 2018;9:338-48. [Crossref] [PubMed]
- Comblain F, Barthélémy N, Lefèbvre M, et al. A randomized, double-blind, prospective, placebo-controlled study of the efficacy of a diet supplemented with curcuminoids extract, hydrolyzed collagen and green tea extract in owner's dogs with osteoarthritis. BMC Vet Res 2017;13:395. [Crossref] [PubMed]
- Xu R, Bai Y, Yang K, et al. Effects of green tea consumption on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond) 2020;17:56. [Crossref] [PubMed]
- Potenza MA, Iacobazzi D, Sgarra L, et al. The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications. Molecules 2020;25:3061. [Crossref] [PubMed]
- Menegazzi M, Campagnari R, Bertoldi M, et al. Protective Effect of Epigallocatechin-3-Gallate (EGCG) in Diseases with Uncontrolled Immune Activation: Could Such a Scenario Be Helpful to Counteract COVID-19? Int J Mol Sci 2020;21:5171. [Crossref] [PubMed]
- Oz HS. Chronic Inflammatory Diseases and Green Tea Polyphenols. Nutrients 2017;9:561. [Crossref] [PubMed]
- Barbalho SM, Bosso H, Salzedas-Pescinini LM, et al. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases. Complement Ther Med 2019;43:148-53. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, w64.
- Almatroodi SA, Almatroudi A, Khan AA, et al. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020;25:3146. [Crossref] [PubMed]
- Doss MX, Potta SP, Hescheler J, et al. Trapping of growth factors by catechins: a possible therapeutical target for prevention of proliferative diseases. J Nutr Biochem 2005;16:259-66. [Crossref] [PubMed]
- Cooper R, Morré DJ, Morré DM. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. J Altern Complement Med 2005;11:521-8. [Crossref] [PubMed]
- Chowdhury A, Sarkar J, Chakraborti T, et al. Protective role of epigallocatechin-3-gallate in health and disease: A perspective. Biomed Pharmacother 2016;78:50-9. [Crossref] [PubMed]
- Ohishi T, Goto S, Monira P, et al. Anti-inflammatory Action of Green Tea. Antiinflamm Antiallergy Agents Med Chem 2016;15:74-90. [Crossref] [PubMed]
- Cavet ME, Harrington KL, Vollmer TR, et al. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis 2011;17:533-42. [PubMed]
- Fechtner S, Singh A, Chourasia M, et al. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol Appl Pharmacol 2017;329:112-20. [Crossref] [PubMed]
- Kim TY, Leem E, Lee JM, et al. Control of Reactive Oxygen Species for the Prevention of Parkinson's Disease: The Possible Application of Flavonoids. Antioxidants (Basel) 2020;9:583. [Crossref] [PubMed]
- Ahmed S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res Ther 2010;12:208. [Crossref] [PubMed]
- Duan L, Liang Y, Xu X, et al. Recent progress on the role of miR-140 in cartilage matrix remodelling and its implications for osteoarthritis treatment. Arthritis Res Ther 2020;22:194. [Crossref] [PubMed]
- Davidson RK, Waters JG, Kevorkian L, et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther 2006;8:R124. [Crossref] [PubMed]
- Rengel Y, Ospelt C, Gay S. Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther 2007;9:221. [Crossref] [PubMed]
- Majumdar MK, Askew R, Schelling S, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum 2007;56:3670-4. [Crossref] [PubMed]
- Lai Y, Bai X, Zhao Y, et al. ADAMTS-7 forms a positive feedback loop with TNF-α in the pathogenesis of osteoarthritis. Ann Rheum Dis 2014;73:1575-84. [Crossref] [PubMed]
- Akuri MC, Barbalho SM, Val RM, et al. Reflections about Osteoarthritis and Curcuma longa. Pharmacogn Rev 2017;11:8-12. [Crossref] [PubMed]
- Győri DS, Mócsai A. Osteoclast Signal Transduction During Bone Metastasis Formation. Front Cell Dev Biol 2020;8:507. [Crossref] [PubMed]
- Karami J, Masoumi M, Khorramdelazad H, et al. Role of autophagy in the pathogenesis of rheumatoid arthritis: Latest evidence and therapeutic approaches. Life Sci 2020;254:117734 [Crossref] [PubMed]
- Kovács B, Vajda E, Nagy EE. Regulatory Effects and Interactions of the Wnt and OPG-RANKL-RANK Signaling at the Bone-Cartilage Interface in Osteoarthritis. Int J Mol Sci 2019;20:4653. [Crossref] [PubMed]
- Samuels JS, Holland L, López M, et al. Prostaglandin E2 and IL-23 interconnects STAT3 and RoRγ pathways to initiate Th17 CD4(+) T-cell development during rheumatoid arthritis. Inflamm Res 2018;67:589-96. [Crossref] [PubMed]
- Egg D. Concentrations of prostaglandins D2, E2, F2 alpha, 6-keto-F1 alpha and thromboxane B2 in synovial fluid from patients with inflammatory joint disorders and osteoarthritis. Z Rheumatol 1984;43:89-96. [PubMed]
- Ahmed S, Rahman A, Hasnain A, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med 2002;33:1097-105. [Crossref] [PubMed]
- Singh R, Ahmed S, Islam N, et al. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum 2002;46:2079-86. [Crossref] [PubMed]
- Hafeez BB, Ahmed S, Wang N, et al. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol Appl Pharmacol 2006;216:11-9. [Crossref] [PubMed]
- Adcocks C, Collin P, Buttle DJ. Catechins from green tea (Camellia sinensis) inhibit bovine and human cartilage proteoglycan and type II collagen degradation in vitro. J Nutr 2002;132:341-6. [Crossref] [PubMed]
- Rasheed Z, Rasheed N, Al-Shaya O. Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1β-stimulated human osteoarthritis chondrocytes: potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur J Nutr 2018;57:917-28. [Crossref] [PubMed]
- Andriamanalijaona R, Kypriotou M, Baugé C, et al. Comparative effects of 2 antioxidants, selenomethionine and epigallocatechin-gallate, on catabolic and anabolic gene expression of articular chondrocytes. J Rheumatol 2005;32:1958-67. [PubMed]
- Comblain F, Dubuc JE, Lambert C, et al. Identification of Targets of a New Nutritional Mixture for Osteoarthritis Management Composed by Curcuminoids Extract, Hydrolyzed Collagen and Green Tea Extract. PLoS One 2016;11:e0156902 [Crossref] [PubMed]
- Hashempur MH, Sadrneshin S, Mosavat SH, et al. Green tea (Camellia sinensis) for patients with knee osteoarthritis: A randomized open-label active-controlled clinical trial. Clin Nutr 2018;37:85-90. [Crossref] [PubMed]
Cite this article as: Barbalho SM, Goulart RDA, Buglio DS, Araujo AC, Guiguer EL. The possible role of green tea on osteoarthritis: a narrative report. Longhua Chin Med 2020;3:11.

