Comparing the physiochemical properties of natural, synthetic and adulterated honeys
Introduction
Honey is a naturally viscous and sweet substance produced by honey bees from the nectar of flowers or secretions of living plants or excretions of plant-sucking insects on plants that the bees collect and transform with their own salivary enzymes, and then deposit in beehive to ripen and mature (1). It has been consumed as food and medicine by mankind since centuries. The demand for honey is getting increasingly higher, mainly due to the limited supply and remarkable benefits of honey consumption. The benefits are predominantly attributed to the therapeutic effects contributed by bioactive compounds in honey, in addition to its high nutritional level. Therefore, honey is susceptible to adulteration with cheaper sweeteners including syrups and molasses, and sometimes added with a cocktail of chemicals to produce desired colour and bubbles.
Many studies have been conducted to detect adulterated honey in recent years. Different advanced analytical techniques such as spectroscopy, chromatography, differential calorimetry, biosensor, and stable carbon isotope ratio mass spectrometry have been applied (2,3). Each of the methods has its advantages and limitations. The detection of fraudulent honey is mostly focused on the sugar profile. Honey adulteration with sugars has also directly altered the physicochemical and rheological properties of honey.
Gebremariam and Brhane (4) and Ribeiro et al. (5) observed the pH value of adulterated honey would increase if commercial sugars such as corn or cane syrups was added into honey. On the other hand, Oroian et al. (6) reported that the adulteration with hydrolyzed inulin syrup which is acidic in nature would decrease the pH value of honey. According to Naila et al. (7), adulterated honey appeared to be brighter, while pure honey would be more reddish. Anyhow, the colour of honey would also be affected during storage. The colour change is strongly dependent on the temperature of storage and moisture content in honey (8).
There were studies revealing that electrical conductivity was one of the reliable parameters to detect fraudulent honey (9). Honey conductivity is one of the characteristics monitored and recommended by the European Communities to be less than 0.8 mS/cm (10). The limit is applied if 20 g honey is diluted with 100 mL water before measurement. The electrical conductivity is the manifestation of ion movement which is strongly influenced by viscosity and temperature. The viscosity of honey was found to be in a broad range of 18,169 to 2,560 cP at 25 oC (11). Hydroxymethylfurfural is another critical parameter to monitor honey adulteration according to the European Communities. This parameter is also regarded as a quality indicator for honey freshness and thermally over-treated during processing. Previous data on hydroxymethylfurfural in honey reported by worldwide researchers had been collected and statistically analysed by Chua (12) who found that the HMF content was exponentially increased if honey was treated at high temperature (90–100 °C), especially for long duration.
The objective of the present study was to compare the physiochemical properties of natural and synthetic honeys, since honey adulteration is getting serious nowadays. A few rapid assays such as pH, colour, electrical conductivity, viscosity, moisture, total soluble solid, antioxidant capacity and hydroxymethylfurfural content of honey samples were conducted on Tualang honey (natural honey) which is the popular honey in Malaysia, laboratory prepared synthetic honey and market purchased honeys for comparison. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/lcm-20-30).
Methods
Honey samples
The natural Tualang honey was purchased from the Federal Agricultural Marketing Authority (Kuala Nerang, Kedah). Two market honeys which were claimed to be Tualang honey were sourced from the local market. One synthetic honey (100 g) was prepared by dissolving glucose (33.5 g), fructose (40.5 g), sucrose (1.5 g) and maltose (7.5 g) in 17 mL deionized water according to the method described by Cooper et al. (13). The synthetic honey was heated at 100 °C for 6 hours until its colour changed to the dark yellow. The loss of water was replaced for the subsequent experiments.
Determination of pH value
The pH of samples was determined according to the method described in the International Honey Commission (14). A digital pH meter (Mettler Toledo Delta 320, Shah Alam, Malaysia) was calibrated at room temperature using pH buffer 4 and 7. A 10 g sample was dissolved in 75 mL distilled water. The solution was well mixed before the reading was recorded.
Determination of electrical conductivity
The electrical conductivity was measured using the method described in the Europe Honey Commission (14). A well-mixed solution consisted of 20 g honey in 100 mL distilled water was measured using a conductivity meter (EcoScan COND 6+, Eutech Instruments, Singapore). The results are expressed in mS/cm.
Determination of colour intensity
Different concentrations of sample ranged from 20–50% w/v was prepared by dilution with distilled water. The sample solution was filtered with a 0.45 µm nylon filter prior to absorbance measurement using a spectrophotometer (UV1800, Shimadzu, Japan) at 450 and 720 nm (15). The difference of absorbance at both wavelengths is expressed as colour intensity in mAU.
Determination of honey viscosity
The viscosity of samples (50% w/v) was measured at 30 and 40 °C using a Brookfield viscometer equipped with a spindle CP42 (model LV, MA, USA).
Determination of moisture content
A 2 g sample was placed on a disk and dried in oven at 105 °C for 16 hours (16). The weight of the sample was recorded after constant weight was achieved. The loss was due to the evaporation of water in sample.
Determination of total soluble solid
The prism of a digital pocket refractometer (Atago Pal-α, China) was cleaned with Kim Wipe. A drop of homogenized honey sample was placed on the prism for measurement. The total soluble solid was measured at 25 °C and the results were expressed in oBrix.
Determination of ferric reducing antioxidant power
The assay was carried out according to the procedures described by Benzie and Strain (17) who used the principle of reduction of a ferric 2,4,6-tripyridyl-s-triazine complex (Fe3+-TPTZ) to its ferrous, coloured form (Fe2+-TPTZ) in the presence of antioxidants. The reagent contained 2.5 mL of 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, 2.5 mL of 20 mM FeCl3 and 25 mL of 0.3 M acetate buffer at pH 3.6. It was prepared daily and warmed to 37 °C. A 200 µL sample (10%w/v) was mixed with 1.8 mL of the reagent and the absorbance of the reaction mixture was measured spectrophotometrically at 593 nm after incubation at 37 °C for 10 min. The standard solutions of FeSO4·7H2O (100–1,000 µM) were used to construct a calibration curve. The results are expressed as µM Fe2+.
Determination of radical scavenging activity
The assay was carried out according to the procedures described by Chua et al. (18) with minor modification. A 100 µL sample (100–600 mg/mL) was mixed with 1.9 mL DPPH (130 µM) in ethanol and 1 mL acetate buffer solution (100 mM, pH 5.5). The mixture was shaken vigorously and left for 90 min at room temperature in the dark place. The absorbance of the solution was measured at 517 nm. The radical scavenging activity was determined based on Eq. [1].
Determination of hydroxymethylfurfural by LC-MS/MS
About 5 g sample was weighed into a 15 mL test tube. The sample was diluted with 5 mL of 0.1% formic acid solution. The solution was stirred for 30 minutes. Then, it was transferred into a separating funnel and added in 10 mL chloroform for liquid-liquid extraction. The solution was left for 30 minutes to form a two layers solution after vigorously shaking. The lower layer of chloroform was transferred out and the remaining solution was washed again with another 10 mL chloroform. The step was repeated three times. The collected chloroform fractions were combined and dried until achieved constant weight. The dried white solid was dissolved in methanol for LC-MS/MS analysis.
A 5 µL sample was injected into an HPLC system (Dionex Corporation Ultimate 3000; Sunnyvale, CA) equipped with a C18 reversed phase XSelect HSS T3 column (2.1 × 100 mm, 2.5 µm) and a QTOF mass spectrometer (AB SCIEX QSTAR Elite; Foster City, CA). A binary gradient system consisted of solvent A (water with 0.1% formic acid) and solvent B (acetonitrile) was used as mobile phase. The mass spectrometer was used to detect the presence of hydroxymethylfurfural using the fragments of m/z 127 (parent ion) and m/z 90 (daughter ion) in the positive ion mode. Nitrogen gas was used for nebulizing (40 psi) and curtain gas (25 psi). The voltage of ion spray was 5,500 V, the declustering potential was 40 V and the focusing potential was set at 250 V.
Results
Table 1 is the physiochemical properties of honey samples. The results show that all honey samples exhibited the acceptable range of pH (3.4–6.1) (19) and total soluble solid (≥75%) (20) in honey. However, synthetic honey was found to be higher pH values than the other honeys. The significant difference of Tualang honey compared to the other samples was its electrical conductivity and hydroxymethylfurfural content. The high electrical conductivity was attributed to the high mineral content of Tualang honey as published in previous studies (21). The synthetic honey and market honeys showed to have lower electrical conductivity which might be contributed by organic acids resulted from degradation of saccharides during preparation. The chemical degradation can also be seen from the high content of hydroxymethylfurfural in market honeys. Possibly, the market honeys were adulterated with sugar solution because its electrical conductivity was much lower than Tualang honey, but the value was just slightly higher than synthetic honey.

Full table
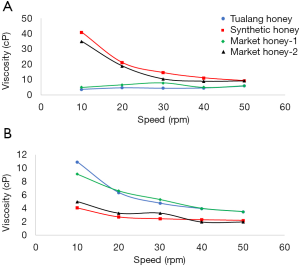
Honey is a viscous substance, usually behaves as Newtonian fluid. The viscosity of honey samples was measured at 30 and 40 °C in this study. The results found that all honey samples could achieve up to around 5,000 cP at 10 rpm with no significant difference among the samples. The honey samples also displayed Newtonian behavior in which the shear stress was linearly increased with the shear rate ranged from 19 to 190 s-1. Similarly, the selected blossom and honeydew honeys from Czech Republic were also reported to be in Newtonian behavior (22). In 2015, researchers from India reported that adulterated honeys would display non-Newtonian behavior (23).
The honey samples were then diluted to 50% w/v and re-measured for their viscosity as presented in Figure 1. Dilution did not change the viscosity of synthetic honey and market honey-2 at 30 °C significantly. Both samples were also found to have the lower moisture content which directly contributed to higher viscosity. However, Tualang honey and market honey-1 was decreased to less than 1,000 cP at 10–50 rpm after dilution with water. The increase of temperature was further decreased the viscosity of Tualang honey and market honey-1 to less than 600 cP and less than 400 cP for synthetic honey and market honey-2. The observation noticed that a large change in the viscosity of synthetic honey compared to Tualng honey at 10 °C increment. The viscosity of synthetic honey was not affected by dilution at 30 °C, but its value was significantly dropped to about 4 to 10 times lower at 40 °C. On the other hand, the viscosity of Tualng honey was just decreased for about 0.5–3 times when the temperature was increased from 30–40 °C. The viscosity of market honeys was in between the values of Tualang honey and synthetic honey. The flow behavior of market honey-1 was near to synthetic honey, whereas market honey-2 was close to Tualang honey.
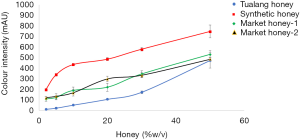
The colour intensity of honey samples at different concentrations is presented in Figure 2. Synthetic honey and Tualang honey shows to have the highest and lowest colour intensity, respectively. Market honeys show to have comparable colour intensity from the concentration of 2–50% w/v.
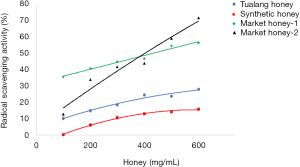
The DPPH assay showed to have an increment of radical scavenging activity when the concentration of honey samples was increased from 100–600 mg/mL (Figure 3). The figure shows that the scavenging activity of Tualang honey was slightly higher than synthetic honey, which was about 10% difference. However, the scavenging activity of both market honeys was much higher than Tualang honey and synthetic honey. This was in line with higher reducing power of market honeys. The high content of hydroxymethylfurfural might explain the high scavenging activity and reducing power of market honeys. Previously, Zhao et al. (24) reported that hydroxymethylfurfural could contribute to the antioxidant activity in a dose dependent manner. Therefore, the antioxidant capacity of honey samples could be contributed by the presence of botanical chemicals and hydroxymethylfurfural in honeys. Hydroxymethylfurfural has been used as a good indicator for honey adulterated with inverted sugars (25). However, this furan derivative could also be produced automatically in honey due to the degradation of reducing sugars in Maillard reaction, particularly after heating and prolonged storage.
Conclusions
Tualang honey was obviously different from synthetic honey in term of its electrical conductivity, colour, viscosity and hydroxymethylfurfural content. The hydroxymethylfurfural content was also contributed to the reducing power and radical scavenging activity of market honeys. Most probably, the market honeys were mixed with sugar solution and the mixing may involve thermal treatment which contributed to high hydroxymethylfurfural content. The electrical conductivity of market honeys was lower than Tualang honey, but higher than synthetic honey. To conclude, no single physiochemical test is capable to differentiate the natural honey and adulterated honey, especially the mixture of both honeys. However, the combination of the quick assays was able to conform the adulteration of honey satisfactory.
Acknowledgments
The authors would like to express their appreciation to Nur Ashikin Othman and other internship students for running antioxidant assays during the semester of 2017/2018 in the university.
Funding: The study was funded by Universiti Teknologi Malaysia (GUP 04H45).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/lcm-20-30
Data Sharing Statement: Available at http://dx.doi.org/10.21037/lcm-20-30
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Food and Agriculture Organization. Food and Agriculture Organization of the United Nations. Revised codex standard for honey (No. CODEX STAN 12-1981), 2001. Accessed February 12, 2017. Available online: www.fao.org/input/download/standards/310/cxs_012e.pdf
- Cordella C, Militao JSLT, Clement MC, et al. Detection and quantification of honey adulteration via direct incorporation of sugar syrups or bee-feeding: preliminary study using high-performance anion exchange chromatography with pulsed amperometric detection(HPAEC-PAD) and chemometrics. Anal Chim Acta 2005;531:239-48. [Crossref]
- Elflein L, Raezke KP. Improved detection of honey adulteration by measuring differences between13C/12C stable carbon isotope ratios of protein and sugar compounds with a combination of elemental analyzer - isotope ratio mass spectrometry and liquid chromatography - isotope ratio mass spectrometry (δ13C-EA/LC-IRMS). Apidologie 2008;39:574-87. [Crossref]
- Gebremariam T, Brhane G. Determination of quality and adulteration effects of honey from Adigrat and its surrounding areas. Int J Technol Enhanc Emerg Eng Res 2014;2:71-6.
- Ribeiro RDOR, Mársico ET, da Silva Carneiro C, et al. Detection of honey adulteration of high fructose corn syrup by low field nuclear magnetic resonance (LF 1H NMR). J Food Eng 2014;135:39-43. [Crossref]
- Oroian M, Ropciuc S, Paduret S. Honey authentication using rheological and physicochemical properties. J Food Sci Technol 2018;55:4711-8. [Crossref] [PubMed]
- Naila A, Flint SH, Sulaiman AZ, et al. Classical and novel approaches to the analysis of honey and detection of adulterants. Food Control 2018;90:152-65. [Crossref]
- Bulut L, Kilic M. Kinetics of hydroxymethylfurfural accumulation and color change in honey during storage in relation to moisture content. J Food Process Pres 2009;33:22-32. [Crossref]
- Corbella E, Cozzolino D. Classification of the floral origin of Uruguayan honeys by chemical and physical characteristics combined with chemometrics. LWT-Food Sci Technol 2006;39:534-9. [Crossref]
- Official Journal of the European Communities (EU Directive 110/2001) as the standard composition of honey Official Journal of the European Communities, 2001. LL 10/47-L 10/52.12.1.2002. Council Directive 2001/110/EC of December 2001 Relating to Honey.
- Rybak-Chmielewska H, Szczesna T. Viscosity of honey. Research Institute of Pomology and Floriculture, Apiculture Division, Pulawy, Poland. 1999.
- Chua LS. The Extent of Hydroxymethylfurfural Formation in Honey by Heating Temperature and Duration. Lett Org Chem 2018;15:233-40. [Crossref]
- Cooper RA, Halas E, Molan PC. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J Burn Care Rehabil 2002;23:366-70. [Crossref] [PubMed]
- Bogdanov S. Harmonized methods of the International Honey Commission. Bee Product Science, 2009. Assessed February 21, 2020. Available online: http://www.ihc-platform.net/ihcmethods2009.pdf
- Beretta G, Granata P, Ferrero M, et al. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta 2005;533:185-91. [Crossref]
- Association of Official Analysis Chemists, method 969.38. Official Methods of Analysis. 15th ed., Washington, USA, 1990.
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘Antioxidant Power”: The FRAP assay. Anal Biochem 1996;239:70-6. [Crossref] [PubMed]
- Chua LS, Rahaman NL, Adnan NA, et al. Antioxidant activity of three honey samples in relation with their biochemical components. J Anal Methods Chem 2013;2013:313798 [Crossref] [PubMed]
- Bogdanov S. Honey control. Book of honey, chapter 9. Bee Product Science, 2010. Assessed February 21, 2020. Available online:https://www.scribd.com/document/62323852/9-Honey-Control
- de Sousa JMB, de Souza EL, Marques G, et al. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region LWT - Food Sci Technol 2016;65:645-51. [Crossref]
- Chua LS, Abdul-Rahaman NL, Sarmidi MR, et al. Multi-element composition and physical properties of honey samples from Malaysia. Food Chem 2012;135:880-7. [Crossref] [PubMed]
- Trávníček P, Vítěz T, Přidal A. Rheological properties of honey. Sci Agric Bohem 2012;43:160-5. [Crossref]
- Kamboj U, Mishra S. Prediction of adulteration in honey using rheological parameters. Int J Food Prop 2015;18:2056-63. [Crossref]
- Zhao L, Chen J, Su J, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J Agric Food Chem 2013;61:10604-11. [Crossref] [PubMed]
- Pasias IN, Kiriakou IK, Proestos C. HMF and diastase activity in honeys: a fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem 2017;229:425-31. [Crossref] [PubMed]
Cite this article as: Chua LS, Hamzah NFA. Comparing the physiochemical properties of natural, synthetic and adulterated honeys. Longhua Chin Med 2020;3:8.